Cryptotanshinone suppresses androgen receptor-mediated growth in androgen dependent and castration resistant prostate cancer cells
- PMID: 22154085
- PMCID: PMC3283034
- DOI: 10.1016/j.canlet.2011.10.006
Cryptotanshinone suppresses androgen receptor-mediated growth in androgen dependent and castration resistant prostate cancer cells
Abstract
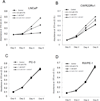
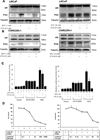
Androgen receptor (AR) is the major therapeutic target for the treatment of prostate cancer (PCa). Anti-androgens to reduce or prevent androgens binding to AR are widely used to suppress AR-mediated PCa growth; however, the androgen depletion therapy is only effective for a short period of time. Here we found a natural product/Chinese herbal medicine cryptotanshinone (CTS), with a structure similar to dihydrotestosterone (DHT), can effectively inhibit the DHT-induced AR transactivation and prostate cancer cell growth. Our results indicated that 0.5 μM CTS effectively suppresses the growth of AR-positive PCa cells, but has little effect on AR negative PC-3 cells and non-malignant prostate epithelial cells. Furthermore, our data indicated that CTS could modulate AR transactivation and suppress the DHT-mediated AR target genes (PSA, TMPRSS2, and TMEPA1) expression in both androgen responsive PCa LNCaP cells and castration resistant CWR22rv1 cells. Importantly, CTS selectively inhibits AR without repressing the activities of other nuclear receptors, including ERα, GR, and PR. The mechanistic studies indicate that CTS functions as an AR inhibitor to suppress androgen/AR-mediated cell growth and PSA expression by blocking AR dimerization and the AR-coregulator complex formation. Furthermore, we showed that CTS effectively inhibits CWR22Rv1 cell growth and expressions of AR target genes in the xenograft animal model. The previously un-described mechanisms of CTS may explain how CTS inhibits the growth of PCa cells and help us to establish new therapeutic concepts for the treatment of PCa.
Copyright © 2011 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
None Declared
Figures





Similar articles
-
The selective inhibitory effect of a synthetic tanshinone derivative on prostate cancer cells.Prostate. 2012 May 15;72(7):803-16. doi: 10.1002/pros.21474. Epub 2011 Sep 19. Prostate. 2012. PMID: 21932429
-
Tanshinones from Chinese medicinal herb Danshen (Salvia miltiorrhiza Bunge) suppress prostate cancer growth and androgen receptor signaling.Pharm Res. 2012 Jun;29(6):1595-608. doi: 10.1007/s11095-012-0670-3. Epub 2012 Jan 27. Pharm Res. 2012. PMID: 22281759
-
Evaluation of the effects of androgenic Chinese herbal medicines on androgen receptors and tumor growth in experimental prostate cancer models.J Ethnopharmacol. 2020 Oct 5;260:113058. doi: 10.1016/j.jep.2020.113058. Epub 2020 Jun 7. J Ethnopharmacol. 2020. PMID: 32525068
-
Androgen action in the prostate gland.Minerva Urol Nefrol. 2012 Mar;64(1):35-49. Minerva Urol Nefrol. 2012. PMID: 22402316 Review.
-
Androgen receptors in hormone-dependent and castration-resistant prostate cancer.Pharmacol Ther. 2013 Dec;140(3):223-38. doi: 10.1016/j.pharmthera.2013.07.003. Epub 2013 Jul 13. Pharmacol Ther. 2013. PMID: 23859952 Review.
Cited by
-
Kaempferol Promotes Apoptosis While Inhibiting Cell Proliferation via Androgen-Dependent Pathway and Suppressing Vasculogenic Mimicry and Invasion in Prostate Cancer.Anal Cell Pathol (Amst). 2019 Dec 1;2019:1907698. doi: 10.1155/2019/1907698. eCollection 2019. Anal Cell Pathol (Amst). 2019. PMID: 31871879 Free PMC article.
-
Bioavailability and pharmacokinetic comparison of tanshinones between two formulations of Salvia miltiorrhiza in healthy volunteers.Sci Rep. 2017 Jul 5;7(1):4709. doi: 10.1038/s41598-017-02747-4. Sci Rep. 2017. PMID: 28680091 Free PMC article.
-
Roles of Reactive Oxygen Species in Anticancer Therapy with Salvia miltiorrhiza Bunge.Oxid Med Cell Longev. 2016;2016:5293284. doi: 10.1155/2016/5293284. Epub 2016 Aug 4. Oxid Med Cell Longev. 2016. PMID: 27579153 Free PMC article.
-
Molecular evidence of cryptotanshinone for treatment and prevention of human cancer.Anticancer Agents Med Chem. 2013 Sep;13(7):979-87. doi: 10.2174/18715206113139990115. Anticancer Agents Med Chem. 2013. PMID: 23272908 Free PMC article. Review.
-
Cryptotanshinone has curative dual anti-proliferative and immunotherapeutic effects on mouse Lewis lung carcinoma.Cancer Immunol Immunother. 2019 Jul;68(7):1059-1071. doi: 10.1007/s00262-019-02326-8. Epub 2019 Apr 10. Cancer Immunol Immunother. 2019. PMID: 30972427 Free PMC article.
References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010, CA. Cancer J Clin. 2010;60:277–300. - PubMed
-
- Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr. Rev. 2004;25(2):276–308. - PubMed
-
- Henderson BE, Feigelson HS. Hormonal carcinogenesis. Carcinogenesis. 2000;21:427–433. - PubMed
-
- Marker PC, Donjacour AA, Dahiya R, Cunha GR. Hormonal, cellular, and molecular control of prostatic development. Dev Biol. 2003;253(2):165–174. - PubMed
-
- Bonkhoff H, Remberger K. Differentiation pathways and histogenetic aspects of normal and abnormal prostatic growth: a stem cell model. The Prostate. 1996;28(2):98–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

