First neuropathological description of a patient with Parkinson's disease and LRRK2 p.N1437H mutation
- PMID: 22154298
- PMCID: PMC3330199
- DOI: 10.1016/j.parkreldis.2011.11.019
First neuropathological description of a patient with Parkinson's disease and LRRK2 p.N1437H mutation
Abstract
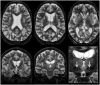
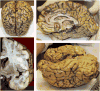
The c.4309A>C mutation in the LRRK2 gene (LRRK2 p.N1437H) has recently been reported as the seventh pathogenic LRRK2 mutation causing monogenic Parkinson's disease (PD). So far, only two families worldwide have been identified with this mutation. By screening DNA from seven brains of PD patients, we found one individual with seemingly sporadic PD and LRRK2 p.N1437H mutation. Clinically, the patient had levodopa-responsive PD with tremor, and developed severe motor fluctuations during a disease duration of 19 years. There was severe and painful ON-dystonia, and severe depression with suicidal thoughts during OFF. In the advanced stage, cognition was slow during motor OFF, but there was no noticeable cognitive decline. There were no signs of autonomic nervous system dysfunction. Bilateral deep brain stimulation of the subthalamic nucleus had unsatisfactory results on motor symptoms. The patient committed suicide. Neuropathological examination revealed marked cell loss and moderate alpha-synuclein positive Lewy body pathology in the brainstem. There was sparse Lewy pathology in the cortex. A striking finding was very pronounced ubiquitin-positive pathology in the brainstem, temporolimbic regions and neocortex. Ubiquitin positivity was most pronounced in the white matter, and was out of proportion to the comparatively weaker alpha-synuclein immunoreactivity. Immunostaining for tau was mildly positive, revealing non-specific changes, but staining for TDP-43 and FUS was entirely negative. The distribution and shape of ubiquitin-positive lesions in this patient differed from the few previously described patients with LRRK2 mutations and ubiquitin pathology, and the ubiquitinated protein substrate remains undefined.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004 Nov 18;44(4):601–7. - PubMed
-
- Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004 Nov 18;44(4):595–600. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

