Quantitative structure-property relationship modeling of remote liposome loading of drugs
- PMID: 22154932
- PMCID: PMC3432270
- DOI: 10.1016/j.jconrel.2011.11.029
Quantitative structure-property relationship modeling of remote liposome loading of drugs
Abstract
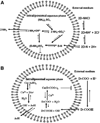
Remote loading of liposomes by trans-membrane gradients is used to achieve therapeutically efficacious intra-liposome concentrations of drugs. We have developed Quantitative Structure Property Relationship (QSPR) models of remote liposome loading for a data set including 60 drugs studied in 366 loading experiments internally or elsewhere. Both experimental conditions and computed chemical descriptors were employed as independent variables to predict the initial drug/lipid ratio (D/L) required to achieve high loading efficiency. Both binary (to distinguish high vs. low initial D/L) and continuous (to predict real D/L values) models were generated using advanced machine learning approaches and 5-fold external validation. The external prediction accuracy for binary models was as high as 91-96%; for continuous models the mean coefficient R(2) for regression between predicted versus observed values was 0.76-0.79. We conclude that QSPR models can be used to identify candidate drugs expected to have high remote loading capacity while simultaneously optimizing the design of formulation experiments.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Liposome drugs' loading efficiency: a working model based on loading conditions and drug's physicochemical properties.J Control Release. 2009 Oct 1;139(1):73-80. doi: 10.1016/j.jconrel.2009.05.036. Epub 2009 Jun 7. J Control Release. 2009. PMID: 19508880
-
Computer-aided design of liposomal drugs: In silico prediction and experimental validation of drug candidates for liposomal remote loading.J Control Release. 2014 Jan 10;173:125-31. doi: 10.1016/j.jconrel.2013.10.029. Epub 2013 Oct 31. J Control Release. 2014. PMID: 24184343 Free PMC article.
-
A drug release study from hydroxypropylmethylcellulose (HPMC) matrices using QSPR modeling.J Pharm Sci. 2007 Dec;96(12):3334-51. doi: 10.1002/jps.20990. J Pharm Sci. 2007. PMID: 17626286
-
The significance of drug-to-lipid ratio to the development of optimized liposomal formulation.J Liposome Res. 2018 Sep;28(3):249-258. doi: 10.1080/08982104.2017.1343836. Epub 2017 Jul 6. J Liposome Res. 2018. PMID: 28627268 Review.
-
Improving ADMET Prediction Accuracy for Candidate Drugs: Factors to Consider in QSPR Modeling Approaches.Curr Top Med Chem. 2024;24(3):222-242. doi: 10.2174/0115680266280005231207105900. Curr Top Med Chem. 2024. PMID: 38083894 Review.
Cited by
-
Evaluation of a Platinum-Acridine Anticancer Agent and Its Liposomal Formulation in an in vivo Model of Lung Adenocarcinoma.ChemMedChem. 2021 Jan 19;16(2):412-419. doi: 10.1002/cmdc.202000637. Epub 2020 Oct 22. ChemMedChem. 2021. PMID: 32975041 Free PMC article.
-
Development and characterization of liposomal formulation of bortezomib.Int J Pharm X. 2019 Mar 23;1:100011. doi: 10.1016/j.ijpx.2019.100011. eCollection 2019 Dec. Int J Pharm X. 2019. PMID: 31517276 Free PMC article.
-
Irinotecan Delivery by Lipid-Coated Mesoporous Silica Nanoparticles Shows Improved Efficacy and Safety over Liposomes for Pancreatic Cancer.ACS Nano. 2016 Feb 23;10(2):2702-15. doi: 10.1021/acsnano.5b07781. Epub 2016 Feb 9. ACS Nano. 2016. PMID: 26835979 Free PMC article.
-
Novel poly(2-oxazoline) block copolymer with aromatic heterocyclic side chains as a drug delivery platform.J Control Release. 2019 Aug 10;307:261-271. doi: 10.1016/j.jconrel.2019.06.037. Epub 2019 Jun 28. J Control Release. 2019. PMID: 31260756 Free PMC article.
-
Immune checkpoint inhibition in syngeneic mouse cancer models by a silicasome nanocarrier delivering a GSK3 inhibitor.Biomaterials. 2021 Feb;269:120635. doi: 10.1016/j.biomaterials.2020.120635. Epub 2020 Dec 28. Biomaterials. 2021. PMID: 33422940 Free PMC article.
References
-
- Wagner V, Dullaart A, Bock AK, Zweck A. The emerging nanomedicine landscape. Nat Biotechnol. 2006;24:1211–1217. - PubMed
-
- Gabizon A, Catane R, Uziely B, Kaufman B, Safra T, Cohen R, Martin F, Huang A, Barenholz Y. Prolonged Circulation Time and Enhanced Accumulation in Malignant Exudates of Doxorubicin Encapsulated in Polyethylene-Glycol Coated Liposomes. Cancer Res. 1994;54:987–992. - PubMed
-
- Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal doxorubicin - Review of animal and human studies. Clin Pharmacokinet. 2003;42:419–436. - PubMed
-
- Barenholz Y. Harnessing biomaterials for Nanomedicine: Preparation, toxicity and applications. Pan Stanford Publishing Pte Ltd; 2011. Doxil®— The First FDA-Approved Nano-Drug: From an Idea to a Product. in Press. www.panstanford.com.
-
- Naparstek Y, Avnir Y, Ulmansky R, Wasserman V, Even-Chen S, Broyer M, Barenholz Y. Amphipathic weak acid glucocorticoid Prodrugs remote-loaded into sterically stabilized nanoliposomes evaluated in arthritic rats and in a beagle dog. Arthritis Rheum. 2008;58:119–129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

