Botulinum toxin in masticatory muscles: short- and long-term effects on muscle, bone, and craniofacial function in adult rabbits
- PMID: 22155510
- PMCID: PMC3278508
- DOI: 10.1016/j.bone.2011.11.015
Botulinum toxin in masticatory muscles: short- and long-term effects on muscle, bone, and craniofacial function in adult rabbits
Abstract
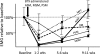
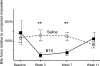
Paralysis of the masticatory muscles using botulinum toxin (BTX) is a common treatment for cosmetic reduction of the masseters as well as for conditions involving muscle spasm and pain. The effects of this treatment on mastication have not been evaluated, and claims that the treatment unloads the jaw joint and mandible have not been validated. If BTX treatment does decrease mandibular loading, osteopenia might ensue as an adverse result. Rabbits received a single dose of BTX or saline into one randomly chosen masseter muscle and were followed for 4 or 12 weeks. Masticatory muscle activity was assessed weekly, and incisor bite force elicited by stimulation of each masseter was measured periodically. At the endpoint, strain gages were installed on the neck of the mandibular condyle and on the molar area of the mandible for in vivo bone strain recording during mastication and muscle stimulation. After termination, muscles were weighed and mandibular segments were scanned with micro CT. BTX paralysis of one masseter did not alter chewing side or rate, in part because of compensation by the medial pterygoid muscle. Masseter-induced bite force was dramatically decreased. Analysis of bone strain data suggested that at 4 weeks, the mandibular condyle of the BTX-injected side was underloaded, as were both sides of the molar area. Bone quantity and quality were severely decreased specifically at these underloaded locations, especially the injection-side condylar head. At 12 weeks, most functional parameters were near their pre-injection levels, but the injected masseter still exhibited atrophy and percent bone area was still low in the condylar head. In conclusion, although the performance of mastication was only minimally harmed by BTX paralysis of the masseter, the resulting underloading was sufficient to cause notable and persistent bone loss, particularly at the temporomandibular joint.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Bilateral treatment of the masseter with botulinum toxin: Consequences for mastication, muscle force and the mandibular condyle.J Oral Rehabil. 2023 Sep;50(9):775-781. doi: 10.1111/joor.13495. Epub 2023 May 24. J Oral Rehabil. 2023. PMID: 37178264
-
Repeated botulinum treatment of rabbit masseter causes cumulative tissue damage.Arch Oral Biol. 2022 Sep;141:105480. doi: 10.1016/j.archoralbio.2022.105480. Epub 2022 Jun 11. Arch Oral Biol. 2022. PMID: 35724521
-
Botulinum toxin in masticatory muscles of the adult rat induces bone loss at the condyle and alveolar regions of the mandible associated with a bone proliferation at a muscle enthesis.Bone. 2015 Aug;77:75-82. doi: 10.1016/j.bone.2015.03.023. Epub 2015 Apr 7. Bone. 2015. PMID: 25857689
-
Correction of Malocclusion by Botulinum Neurotoxin Injection into Masticatory Muscles.Toxins (Basel). 2018 Jan 2;10(1):27. doi: 10.3390/toxins10010027. Toxins (Basel). 2018. PMID: 29301317 Free PMC article. Review.
-
Symphyseal fusion and jaw-adductor muscle force: an EMG study.Am J Phys Anthropol. 2000 Aug;112(4):469-92. doi: 10.1002/1096-8644(200008)112:4<469::AID-AJPA5>3.0.CO;2-V. Am J Phys Anthropol. 2000. PMID: 10918125 Review.
Cited by
-
Chewed out: an experimental link between food material properties and repetitive loading of the masticatory apparatus in mammals.PeerJ. 2015 Nov 3;3:e1345. doi: 10.7717/peerj.1345. eCollection 2015. PeerJ. 2015. PMID: 26557436 Free PMC article.
-
Effect of bisphosphonate on temporomandibular joint in osteopenia-induced rats by botulinum toxin A injection on masticatory muscle: a preliminary study.Maxillofac Plast Reconstr Surg. 2019 Mar 4;41(1):11. doi: 10.1186/s40902-019-0193-5. eCollection 2019 Dec. Maxillofac Plast Reconstr Surg. 2019. PMID: 30915317 Free PMC article.
-
Mandibular Vertical Growth Deficiency After Botulinum-Induced Hypotrophy of Masticatory Closing Muscles in Juvenile Nonhuman Primates.Front Physiol. 2019 Apr 26;10:496. doi: 10.3389/fphys.2019.00496. eCollection 2019. Front Physiol. 2019. PMID: 31080418 Free PMC article.
-
Muscle and joint mechanics during maximum force biting following total temporomandibular joint replacement surgery.Biomech Model Mechanobiol. 2024 Jun;23(3):809-823. doi: 10.1007/s10237-023-01807-1. Epub 2024 Mar 19. Biomech Model Mechanobiol. 2024. PMID: 38502434 Free PMC article.
-
Platelet-rich plasma inhibits RANKL-induced osteoclast differentiation through activation of Wnt pathway during bone remodeling.Int J Mol Med. 2018 Feb;41(2):729-738. doi: 10.3892/ijmm.2017.3258. Epub 2017 Nov 16. Int J Mol Med. 2018. PMID: 29207140 Free PMC article.
References
-
- Fehlings D, Novak I, Berweck S, Hoare B, Stott NS, Russo RN. Botulinum toxin assessment, intervention and follow-up for paediatric upper limb hypertonicity: international consensus statement. Eur J Neurol. 2010;17(Suppl 2):38–56. - PubMed
-
- Kim NH, Chung JH, Park RH, Park JB. The use of botulinum toxin type A in aesthetic mandibular contouring. Plast Reconstr Surg. 2005;115:919–30. - PubMed
-
- Kim NH, Park RH, Park JB. Botulinum toxin type A for the treatment of hypertrophy of the masseter muscle. Plast Reconstr Surg. 2010;125:1693–705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

