Paclitaxel in tyrosine-derived nanospheres as a potential anti-cancer agent: in vivo evaluation of toxicity and efficacy in comparison with paclitaxel in Cremophor
- PMID: 22155544
- PMCID: PMC5953571
- DOI: 10.1016/j.ejps.2011.11.017
Paclitaxel in tyrosine-derived nanospheres as a potential anti-cancer agent: in vivo evaluation of toxicity and efficacy in comparison with paclitaxel in Cremophor
Abstract
Paclitaxel (PTX) has gained widespread clinical use yet its administration is associated with significant toxicity. In the present study, the toxicity and anti-tumor efficacy of tyrosine-derived nanospheres (NSP) for the delivery of PTX was compared to a clinical formulation of PTX in PBS-diluted Cremophor® EL (PTX-CrEL-D). Maximum tolerated dose was determined using a concentration series of PTX in NSP and CrEL-D, with toxicity assessed by measuring changes in body weight. Healthy mice administered PTX-NSP continued to gain weight normally while treatment with PTX-CrEL-D resulted in significant weight loss that failed to recover following treatment. Even at the dose of 50mg/kg, PTX-NSP showed better tolerance than 25mg/kg of PTX-CrEL-D. Xenograft studies of breast cancer revealed that the anti-tumor efficacy of PTX-NSP was equal to that of PTX-CrEL-D in tumors originating from both MDA-MB-435 and ZR-75-1 cancer lines. Larger volume of distribution and longer half-life were measured for PTX-NSP administration compared to those reported in the literature for a CrEL formulation. This trend suggests the potential for improved therapeutic index of PTX when administered via NSP. The findings reported here confirm that the NSP formulation is an efficient method for PTX administration with significant increase in maximum tolerated dose, offering possible clinical implications in the treatment of breast tumors.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures
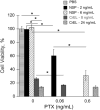
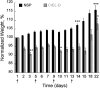

 ) PTX-NSP; (
) PTX-NSP; (
 ) PTX-CrEL-D. (F) 40 (
) PTX-CrEL-D. (F) 40 (
 ) and 50 (
) and 50 (
 ) mg/kg of PTX in NSP. Concentration of NSP and CrEL-D in each treatment was 13 and 52 mg/0.2 mL, respectively. Lethal toxicity (5-10% mortality) was observed in all PTX-CrEL-D treatments, regardless of PTX dose, by the time of the third treatment. The statistical data is expressed as the mean value ± S.E. (standard error); p < 0.05 (*), 0.01 (**), and 0.001 (***) were considered to be statistically significant in the body weight change compared to the initial pre-treatment weight.
) mg/kg of PTX in NSP. Concentration of NSP and CrEL-D in each treatment was 13 and 52 mg/0.2 mL, respectively. Lethal toxicity (5-10% mortality) was observed in all PTX-CrEL-D treatments, regardless of PTX dose, by the time of the third treatment. The statistical data is expressed as the mean value ± S.E. (standard error); p < 0.05 (*), 0.01 (**), and 0.001 (***) were considered to be statistically significant in the body weight change compared to the initial pre-treatment weight.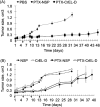

Similar articles
-
Novel biocompatible intraperitoneal drug delivery system increases tolerability and therapeutic efficacy of paclitaxel in a human ovarian cancer xenograft model.Cancer Chemother Pharmacol. 2007 Nov;60(6):907-14. doi: 10.1007/s00280-007-0449-0. Epub 2007 Mar 21. Cancer Chemother Pharmacol. 2007. PMID: 17375303
-
Functionalized nanospheres for targeted delivery of paclitaxel.J Control Release. 2013 Nov 10;171(3):315-21. doi: 10.1016/j.jconrel.2013.06.017. Epub 2013 Jun 20. J Control Release. 2013. PMID: 23792807
-
Encapsulating paclitaxel in polymeric nanomicelles increases antitumor activity and prevents peripheral neuropathy.Biomed Pharmacother. 2020 Dec;132:110864. doi: 10.1016/j.biopha.2020.110864. Epub 2020 Nov 3. Biomed Pharmacother. 2020. PMID: 33254426
-
Albumin-bound paclitaxel: a next-generation taxane.Expert Opin Pharmacother. 2006 Jun;7(8):1041-53. doi: 10.1517/14656566.7.8.1041. Expert Opin Pharmacother. 2006. PMID: 16722814 Review.
-
Alternative formulations of paclitaxel.Cancer Treat Rev. 1997 Mar;23(2):87-95. doi: 10.1016/s0305-7372(97)90022-0. Cancer Treat Rev. 1997. PMID: 9225960 Review.
Cited by
-
Synthesis and characterization of Fatty acid/amino Acid self-assemblies.J Funct Biomater. 2014 Oct 24;5(4):211-31. doi: 10.3390/jfb5040211. J Funct Biomater. 2014. PMID: 25347356 Free PMC article.
-
Nanoparticles and nanofibers for topical drug delivery.J Control Release. 2016 Oct 28;240:77-92. doi: 10.1016/j.jconrel.2015.10.049. Epub 2015 Oct 28. J Control Release. 2016. PMID: 26518723 Free PMC article. Review.
-
NIR-/pH-Responsive drug delivery of functionalized single-walled carbon nanotubes for potential application in cancer chemo-photothermal therapy.Pharm Res. 2013 Nov;30(11):2757-71. doi: 10.1007/s11095-013-1095-3. Epub 2013 Jun 14. Pharm Res. 2013. PMID: 23765399
-
Formulation Strategy for the Delivery of Cyclosporine A: Comparison of Two Polymeric Nanospheres.Sci Rep. 2015 Aug 13;5:13065. doi: 10.1038/srep13065. Sci Rep. 2015. PMID: 26268451 Free PMC article.
-
Development of paclitaxel-TyroSpheres for topical skin treatment.J Control Release. 2012 Oct 10;163(1):18-24. doi: 10.1016/j.jconrel.2012.06.021. Epub 2012 Jun 23. J Control Release. 2012. PMID: 22732474 Free PMC article.
References
-
- Adams JD, Flora KP, Goldspiel BR, Wilson JW, Arbuck SG, Finley R. Taxol: a history of pharmaceutical development and current pharmaceutical concerns. J Natl Cancer Inst Monogr. 1993:141–147. - PubMed
-
- Ahmed F, Pakunlu RI, Brannan A, Bates F, Minko T, Discher DE. Biodegradable polymersomes loaded with both paclitaxel and doxorubicin permeate and shrink tumors, inducing apoptosis in proportion to accumulated drug. J Controlled Release. 2006;116:150–158. - PubMed
-
- Allen C, Maysinger D, Eisenberg A. Nano-engineering block copolymer aggregates for drug delivery. Colloids Surf B: Biointerfaces. 1999;16:3–27.
-
- Bardelmeijer HA, Ouwehand M, Malingre MM, Schellens JH, Beijnen JH, van Tellingen O. Entrapment by Cremophor EL decreases the absorption of paclitaxel from the gut. Cancer Chemother Pharmacol. 2002;49:119–125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

