The plastid outer envelope protein OEP16 affects metabolic fluxes during ABA-controlled seed development and germination
- PMID: 22155670
- PMCID: PMC3295387
- DOI: 10.1093/jxb/err375
The plastid outer envelope protein OEP16 affects metabolic fluxes during ABA-controlled seed development and germination
Abstract
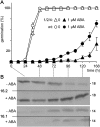
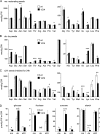
Previously, the OEP16.1 channel pore in the outer envelope membrane of mature pea (Pisum sativum) chloroplasts in vitro has been characterized to be selective for amino acids. Isolation of OEP16.2, a second OEP16 isoform from pea, in the current study allowed membrane localization and gene expression of OEP16 to be followed throughout seed development and germination of Arabidopsis thaliana and P. sativum. Thereby it can be shown on the transcript and protein level that the isoforms OEP16.1 and OEP16.2 in both plant species are alternating: whereas OEP16.1 is prominent in early embryo development and first leaves of the growing plantlet, OEP16.2 dominates in late seed development stages, which are associated with dormancy and desiccation, as well as early germination events. Further, OEP16.2 expression in seeds is under control of the phytohormone abscisic acid (ABA), leading to an ABA-hypersensitive phenotype of germinating oep16 knockout mutants. In consequence, the loss of OEP16 causes metabolic imbalance, in particular that of amino acids during seed development and early germination. It is thus concluded that in vivo OEP16 most probably functions in shuttling amino acids across the outer envelope of seed plastids.
Figures

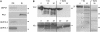




Similar articles
-
Arabidopsis thaliana DOF6 negatively affects germination in non-after-ripened seeds and interacts with TCP14.J Exp Bot. 2012 Mar;63(5):1937-49. doi: 10.1093/jxb/err388. Epub 2011 Dec 8. J Exp Bot. 2012. PMID: 22155632 Free PMC article.
-
A rice orthologue of the ABA receptor, OsPYL/RCAR5, is a positive regulator of the ABA signal transduction pathway in seed germination and early seedling growth.J Exp Bot. 2012 Jan;63(2):1013-24. doi: 10.1093/jxb/err338. Epub 2011 Nov 9. J Exp Bot. 2012. PMID: 22071266
-
Identification of two protein kinases required for abscisic acid regulation of seed germination, root growth, and gene expression in Arabidopsis.Plant Cell. 2007 Feb;19(2):485-94. doi: 10.1105/tpc.106.048538. Epub 2007 Feb 16. Plant Cell. 2007. PMID: 17307925 Free PMC article.
-
First off the mark: early seed germination.J Exp Bot. 2011 Jun;62(10):3289-309. doi: 10.1093/jxb/err030. Epub 2011 Mar 23. J Exp Bot. 2011. PMID: 21430292 Review.
-
Progress of ABA function in endosperm cellularization and storage product accumulation.Plant Cell Rep. 2024 Nov 20;43(12):287. doi: 10.1007/s00299-024-03378-6. Plant Cell Rep. 2024. PMID: 39565413 Review.
Cited by
-
Unknown components of the plastidial permeome.Front Plant Sci. 2014 Aug 19;5:410. doi: 10.3389/fpls.2014.00410. eCollection 2014. Front Plant Sci. 2014. PMID: 25191333 Free PMC article. Review.
-
Bidirectional promoters in seed development and related hormone/stress responses.BMC Plant Biol. 2013 Nov 22;13:187. doi: 10.1186/1471-2229-13-187. BMC Plant Biol. 2013. PMID: 24261334 Free PMC article.
-
Identification of a plastidial phenylalanine exporter that influences flux distribution through the phenylalanine biosynthetic network.Nat Commun. 2015 Sep 10;6:8142. doi: 10.1038/ncomms9142. Nat Commun. 2015. PMID: 26356302 Free PMC article.
-
OEP40, a Regulated Glucose-permeable β-Barrel Solute Channel in the Chloroplast Outer Envelope Membrane.J Biol Chem. 2016 Aug 19;291(34):17848-60. doi: 10.1074/jbc.M115.712398. Epub 2016 Jun 23. J Biol Chem. 2016. PMID: 27339897 Free PMC article.
-
Metabolic, transcriptomic, and genetic analyses of candidate genes for seed size in watermelon.Front Plant Sci. 2024 Jul 16;15:1394724. doi: 10.3389/fpls.2024.1394724. eCollection 2024. Front Plant Sci. 2024. PMID: 39081518 Free PMC article.
References
-
- Angelovici R, Fait A, Fernie AR, Galili G. A seed high-lysine trait is negatively associated with the TCA cycle and slows down Arabidopsis seed germination. New Phytologist. 2011;189:148–159. - PubMed
-
- Angelovici R, Galili G, Fernie AR, Fait A. Seed desiccation: a bridge between maturation and germination. Trends in Plant Science. 2010;15:211–218. - PubMed
-
- Bassel GW, Lan H, Glaab E, Gibbs DJ, Gerjets T, Krasnogor N, Bonner AJ, Holdsworth MJ, Provart NJ. Genome-wide network model capturing seed germination reveals coordinated regulation of plant cellular phase transitions. Proceedings of the National Academy of Sciences, USA. 2011;108:9709–9714. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

