Immunogenicity of the quadrivalent human papillomavirus (type 6/11/16/18) vaccine in males 16 to 26 years old
- PMID: 22155768
- PMCID: PMC3272915
- DOI: 10.1128/CVI.05208-11
Immunogenicity of the quadrivalent human papillomavirus (type 6/11/16/18) vaccine in males 16 to 26 years old
Abstract
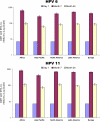
Human papillomavirus (HPV) infection can lead to significant disease in males, including anogenital warts, intraepithelial neoplasias, and several types of oral and anogenital cancers. The quadrivalent HPV (type 6/11/16/18) L1 virus-like particle (VLP) vaccine (qHPV vaccine; Gardasil) has recently been demonstrated to prevent persistent infection and associated disease related to vaccine HPV types in males. We report the overall immunogenicity results from a trial of the quadrivalent HPV vaccine in males. Overall, 3,463 heterosexual men and 602 men who had sex with men were enrolled into a randomized, placebo-controlled, double-blind safety, immunogenicity, and efficacy study. Serum samples were collected prior to vaccination at day 1 and at months 7, 24, and 36 postvaccination. Immunogenicity was evaluated with a multiplex, competitive Luminex immunoassay. Almost all subjects (97.4 to 99.2%) seroconverted for vaccine HPV types by month 7. At month 36, 88.9%, 94.0%, 97.9%, and 57.0% of subjects were still seropositive for HPV-6, -11, -16, and -18, respectively. For all vaccine HPV types, black subjects had significantly higher antibody titers at month 7 than did both Caucasian and Asian subjects. An anamnestic antibody response was seen in men seropositive before vaccination. The vaccine was highly immunogenic in males 16 to 23 years of age; responses were comparable to those observed in women. Furthermore, the immune responses were consistent with the established efficacy of the vaccine in the prevention of incident and persistent HPV infection, anogenital warts, and anal intraepithelial neoplasia.
Figures
References
-
- Castellsague X, Bosch FX, Munoz N. 2003. The male role in cervical cancer. Salud. Publica Mex. 45(Suppl. 3):S345–S353 - PubMed
-
- Cook IF. 2008. Sexual dimorphism of humoral immunity with human vaccines. Vaccine 26:3551–3555 - PubMed
-
- FUTURE II Study Group 2007. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N. Engl. J. Med. 356:1915–1927 - PubMed
-
- Garland SM, et al. 2007. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N. Engl. J. Med. 356:1928–1943 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical