The translocation domain in trimeric autotransporter adhesins is necessary and sufficient for trimerization and autotransportation
- PMID: 22155776
- PMCID: PMC3272944
- DOI: 10.1128/JB.05322-11
The translocation domain in trimeric autotransporter adhesins is necessary and sufficient for trimerization and autotransportation
Abstract
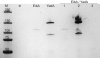
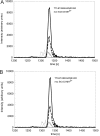
Trimeric autotransporter adhesins (TAAs) comprise one of the secretion pathways of the type V secretion system. The mechanism of their translocation across the outer membrane remains unclear, but it most probably occurs by the formation of a hairpin inside the β-barrel translocation unit, leading to transportation of the passenger domain from the C terminus to the N terminus through the lumen of the β-barrel. We further investigated the phenomenon of autotransportation and the rules that govern it. We showed by coexpressing different Escherichia coli immunoglobulin-binding (Eib) proteins that highly similar TAAs could form stochastically mixed structures (heterotrimers). We further investigated this phenomenon by coexpressing two more distantly related TAAs, EibA and YadA. These, however, did not form heterotrimers; indeed, coexpression was lethal to the cells, leading to elimination of one or another of the genes. However, substituting in either protein the barrel of the other one so that the barrels were identical led to formation of heterotrimers as for Eibs. Our work shows that trimerization of the β-barrel, but not the passenger domain, is necessary and sufficient for TAA secretion while the passenger domain is not.
Figures
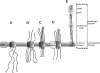



References
-
- Barenkamp SJ, St. Geme JW., III 1996. Identification of a second family of high-molecular-weight adhesion proteins expressed by non-typable Haemophilus influenzae. Mol. Microbiol. 19:1215–1223 - PubMed
-
- Cotter SE, Surana NK, St. Geme JW., III 2005. Trimeric autotransporters: a distinct subfamily of autotransporter proteins. Trends Microbiol. 13:199–205 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

