Systemic cholecystokinin amplifies vago-vagal reflex responses recorded in vagal motor neurones
- PMID: 22155934
- PMCID: PMC3379706
- DOI: 10.1113/jphysiol.2011.224477
Systemic cholecystokinin amplifies vago-vagal reflex responses recorded in vagal motor neurones
Abstract
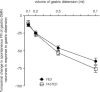
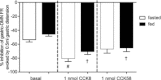
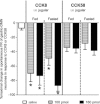
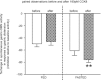
Cholecystokinin (CCK) is a potent regulator of visceral functions as a consequence of its actions on vago-vagal reflex circuit elements. This paper addresses three current controversies regarding the role of CCK to control gastric function via vago-vagal reflexes. Specifically: (a) whether CNS vs. peripheral (vagal afferent) receptors are dominant, (b) whether the long (58) vs. short (8) isoform is more potent and (c) whether nutritional status impacts the gain or even the direction of vago-vagal reflexes. Our in vivo recordings of physiologically identified gastric vagal motor neurones (gastric-DMN) involved in the gastric accommodation reflex (GAR) show unequivocally that: (a) receptors in the coeliac-portal circulation are more sensitive in amplifying gastric vagal reflexes; (b) in the periphery, CCK8 is more potent than CCK58; and (c) the nutritional status has a marginal effect on gastric reflex control. While the GAR reflex is more sensitive in the fasted rat, CCK amplifies this sensitivity. Thus, our results are in stark contrast to recent reports which have suggested that vago-vagal reflexes are inverted by the metabolic status of the animal and that this inversion could be mediated by CCK within the CNS.
Figures



Similar articles
-
Effects of brain stem cholecystokinin-8s on gastric tone and esophageal-gastric reflex.Am J Physiol Gastrointest Liver Physiol. 2009 Mar;296(3):G621-31. doi: 10.1152/ajpgi.90567.2008. Epub 2009 Jan 8. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 19136379 Free PMC article.
-
Impact of antral mechanoreceptor activation on the vago-vagal reflex in the rat: functional zonation of responses.J Physiol. 1992;453:401-11. doi: 10.1113/jphysiol.1992.sp019235. J Physiol. 1992. PMID: 1464835 Free PMC article.
-
Involvement of glutamate in gastrointestinal vago-vagal reflexes initiated by gastrointestinal distention in the rat.Auton Neurosci. 2003 Jan 31;103(1-2):19-37. doi: 10.1016/s1566-0702(02)00145-5. Auton Neurosci. 2003. PMID: 12531396
-
Musings on the wanderer: what's new in our understanding of vago-vagal reflexes? III. Activity-dependent plasticity in vago-vagal reflexes controlling the stomach.Am J Physiol Gastrointest Liver Physiol. 2003 Feb;284(2):G180-7. doi: 10.1152/ajpgi.00413.2002. Am J Physiol Gastrointest Liver Physiol. 2003. PMID: 12529266 Free PMC article. Review.
-
Integration of postprandial function in the proximal gastrointestinal tract. Role of CCK and sensory pathways.Ann N Y Acad Sci. 1994 Mar 23;713:143-56. doi: 10.1111/j.1749-6632.1994.tb44061.x. Ann N Y Acad Sci. 1994. PMID: 8185155 Review.
Cited by
-
Astrocytes in the hindbrain detect glucoprivation and regulate gastric motility.Auton Neurosci. 2013 Apr;175(1-2):61-9. doi: 10.1016/j.autneu.2012.12.006. Epub 2013 Jan 10. Auton Neurosci. 2013. PMID: 23313342 Free PMC article. Review.
-
CXCL12 sensitizes vago-vagal reflex neurons in the dorsal medulla.Brain Res. 2013 Jan 25;1492:46-52. doi: 10.1016/j.brainres.2012.11.022. Epub 2012 Nov 23. Brain Res. 2013. PMID: 23178697 Free PMC article.
-
The gut-brain axis in early Parkinson's disease: from prodrome to prevention.J Neurol. 2025 May 21;272(6):413. doi: 10.1007/s00415-025-13138-5. J Neurol. 2025. PMID: 40394204 Free PMC article. Review.
-
Hindbrain glucoprivation effects on gastric vagal reflex circuits and gastric motility in the rat are suppressed by the astrocyte inhibitor fluorocitrate.J Neurosci. 2014 Aug 6;34(32):10488-96. doi: 10.1523/JNEUROSCI.1406-14.2014. J Neurosci. 2014. PMID: 25100584 Free PMC article.
References
-
- Andresen MC, Yang MY. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am J Physiol Heart Circ Physiol. 1990;259:H1307–H1311. - PubMed
-
- Berthoud HR, Patterson LM. Anatomical relationship between vagal afferent fibers and CCK-immunoreactive entero-endocrine cells in the rat small intestinal mucosa. Acta Anat (Basel) 1996;156:123–131. - PubMed
-
- Buelke-Sam J, Holson JF, Bazare JJ, Young JF. Comparative stability of physiological parameters during sustained anesthesia in rats. Lab Anim Sci. 1978;28:157–162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

