A systematic review to evaluate the accuracy of electronic adverse drug event detection
- PMID: 22155974
- PMCID: PMC3240767
- DOI: 10.1136/amiajnl-2011-000454
A systematic review to evaluate the accuracy of electronic adverse drug event detection
Abstract
Objective: Adverse drug events (ADEs), defined as adverse patient outcomes caused by medications, are common and difficult to detect. Electronic detection of ADEs is a promising method to identify ADEs. We performed this systematic review to characterize established electronic detection systems and their accuracy.
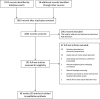
Methods: We identified studies evaluating electronic ADE detection from the MEDLINE and EMBASE databases. We included studies if they contained original data and involved detection of electronic triggers using information systems. We abstracted data regarding rule characteristics including type, accuracy, and rationale.
Results: Forty-eight studies met our inclusion criteria. Twenty-four (50%) studies reported rule accuracy but only 9 (18.8%) utilized a proper gold standard (chart review in all patients). Rule accuracy was variable and often poor (range of sensitivity: 40%-94%; specificity: 1.4%-89.8%; positive predictive value: 0.9%-64%). 5 (10.4%) studies derived or used detection rules that were defined by clinical need or the underlying ADE prevalence. Detection rules in 8 (16.7%) studies detected specific types of ADEs.
Conclusion: Several factors led to inaccurate ADE detection algorithms, including immature underlying information systems, non-standard event definitions, and variable methods for detection rule validation. Few ADE detection algorithms considered clinical priorities. To enhance the utility of electronic detection systems, there is a need to systematically address these factors.
Conflict of interest statement
Figures
References
-
- Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA 1995;274:29–34 - PubMed
-
- Thurmann PA, Thurmann PA. Methods and systems to detect adverse drug reactions in hospitals. Drug Saf 2001;24:961–8 - PubMed
-
- Classen DC, Pestotnik SL, Evans RS, et al. Computerized surveillance of adverse drug events in hospital patients. JAMA 1991;266:2847–51 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous