In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology
- PMID: 22156199
- PMCID: PMC3248292
- DOI: 10.1172/JCI58753
In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology
Abstract
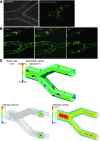
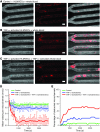
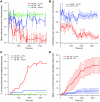
In hematologic diseases, such as sickle cell disease (SCD) and hemolytic uremic syndrome (HUS), pathological biophysical interactions among blood cells, endothelial cells, and soluble factors lead to microvascular occlusion and thrombosis. Here, we report an in vitro "endothelialized" microfluidic microvasculature model that recapitulates and integrates this ensemble of pathophysiological processes. Under controlled flow conditions, the model enabled quantitative investigation of how biophysical alterations in hematologic disease collectively lead to microvascular occlusion and thrombosis. Using blood samples from patients with SCD, we investigated how the drug hydroxyurea quantitatively affects microvascular obstruction in SCD, an unresolved issue pivotal to understanding its clinical efficacy in such patients. In addition, we demonstrated that our microsystem can function as an in vitro model of HUS and showed that shear stress influences microvascular thrombosis/obstruction and the efficacy of the drug eptifibatide, which decreases platelet aggregation, in the context of HUS. These experiments establish the versatility and clinical relevance of our microvasculature-on-a-chip model as a biophysical assay of hematologic pathophysiology as well as a drug discovery platform.
Figures


Similar articles
-
Endothelialized microfluidics for studying microvascular interactions in hematologic diseases.J Vis Exp. 2012 Jun 22;(64):3958. doi: 10.3791/3958. J Vis Exp. 2012. PMID: 22760254 Free PMC article.
-
Analyzing cell mechanics in hematologic diseases with microfluidic biophysical flow cytometry.Lab Chip. 2008 Jul;8(7):1062-70. doi: 10.1039/b802931h. Epub 2008 Jun 5. Lab Chip. 2008. PMID: 18584080 Free PMC article.
-
Applications of microfluidic chip technology in microvascular thrombosis research.Mikrochim Acta. 2025 May 24;192(6):371. doi: 10.1007/s00604-025-07239-1. Mikrochim Acta. 2025. PMID: 40413353 Review.
-
Extracellular fluid tonicity impacts sickle red blood cell deformability and adhesion.Blood. 2017 Dec 14;130(24):2654-2663. doi: 10.1182/blood-2017-04-780635. Epub 2017 Oct 4. Blood. 2017. PMID: 28978568 Free PMC article.
-
The kidney in hematologic disease. A review.Int J Pediatr Nephrol. 1986 Oct-Dec;7(4):207-12. Int J Pediatr Nephrol. 1986. PMID: 3546173 Review.
Cited by
-
Straight Channel Microfluidic Chips for the Study of Platelet Adhesion under Flow.Bio Protoc. 2019 Mar 20;9(6):e3195. doi: 10.21769/BioProtoc.3195. eCollection 2019 Mar 20. Bio Protoc. 2019. PMID: 33654994 Free PMC article.
-
Vascularized platforms for investigating cell communication via extracellular vesicles.Biomicrofluidics. 2024 Sep 23;18(5):051504. doi: 10.1063/5.0220840. eCollection 2024 Sep. Biomicrofluidics. 2024. PMID: 39323481 Review.
-
Thrombosis Models: An Overview of Common In Vivo and In Vitro Models of Thrombosis.Int J Mol Sci. 2023 Jan 29;24(3):2569. doi: 10.3390/ijms24032569. Int J Mol Sci. 2023. PMID: 36768891 Free PMC article. Review.
-
Model Systems to Study the Mechanism of Vascular Aging.Int J Mol Sci. 2023 Oct 19;24(20):15379. doi: 10.3390/ijms242015379. Int J Mol Sci. 2023. PMID: 37895059 Free PMC article. Review.
-
Emerging science of hydroxyurea therapy for pediatric sickle cell disease.Pediatr Res. 2014 Jan;75(1-2):196-204. doi: 10.1038/pr.2013.227. Epub 2013 Nov 19. Pediatr Res. 2014. PMID: 24252885 Free PMC article. Review.
References
-
- Ballas SK, Mohandas N. Sickle red cell microrheology and sickle blood rheology. Microcirculation. 2004;11(2):209–225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

