Macrophages and cathepsin proteases blunt chemotherapeutic response in breast cancer
- PMID: 22156207
- PMCID: PMC3243057
- DOI: 10.1101/gad.180331.111
Macrophages and cathepsin proteases blunt chemotherapeutic response in breast cancer
Abstract
The microenvironment is known to critically modulate tumor progression, yet its role in regulating treatment response is poorly understood. Here we found increased macrophage infiltration and cathepsin protease levels in mammary tumors following paclitaxel (Taxol) chemotherapy. Cathepsin-expressing macrophages protected against Taxol-induced tumor cell death in coculture, an effect fully reversed by cathepsin inhibition and mediated partially by cathepsins B and S. Macrophages were also found to protect against tumor cell death induced by additional chemotherapeutics, specifically etoposide and doxorubicin. Combining Taxol with cathepsin inhibition in vivo significantly enhanced efficacy against primary and metastatic tumors, supporting the therapeutic relevance of this effect. Additionally incorporating continuous low-dose cyclophosphamide dramatically impaired tumor growth and metastasis and improved survival. This study highlights the importance of integrated targeting of the tumor and its microenvironment and implicates macrophages and cathepsins in blunting chemotherapeutic response.
Figures

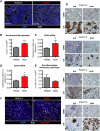
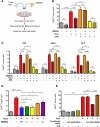
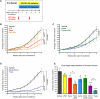


Comment in
-
Microenvironment: Protective surroundings.Nat Rev Cancer. 2011 Dec 23;12(1):2. doi: 10.1038/nrc3196. Nat Rev Cancer. 2011. PMID: 22193399 No abstract available.
References
-
- Bell-McGuinn KM, Garfall AL, Bogyo M, Hanahan D, Joyce JA 2007. Inhibition of cysteine cathepsin protease activity enhances chemotherapy regimens by decreasing tumor growth and invasiveness in a mouse model of multistage cancer. Cancer Res 67: 7378–7385 - PubMed
-
- Blansfield JA, Caragacianu D, Alexander HR III, Tangrea MA, Morita SY, Lorang D, Schafer P, Muller G, Stirling D, Royal RE, et al. 2008. Combining agents that target the tumor microenvironment improves the efficacy of anticancer therapy. Clin Cancer Res 14: 270–280 - PubMed
-
- Bogyo M, Verhelst S, Bellingard-Dubouchaud V, Toba S, Greenbaum D 2000. Selective targeting of lysosomal cysteine proteases with radiolabeled electrophilic substrate analogs. Chem Biol 7: 27–38 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
