Restriction of histone gene transcription to S phase by phosphorylation of a chromatin boundary protein
- PMID: 22156209
- PMCID: PMC3243059
- DOI: 10.1101/gad.173427.111
Restriction of histone gene transcription to S phase by phosphorylation of a chromatin boundary protein
Abstract
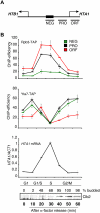
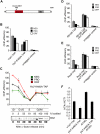
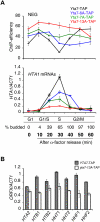
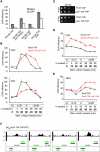
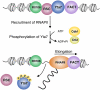
The cell cycle-regulated expression of core histone genes is required for DNA replication and proper cell cycle progression in eukaryotic cells. Although some factors involved in histone gene transcription are known, the molecular mechanisms that ensure proper induction of histone gene expression during S phase remain enigmatic. Here we demonstrate that S-phase transcription of the model histone gene HTA1 in yeast is regulated by a novel attach-release mechanism involving phosphorylation of the conserved chromatin boundary protein Yta7 by both cyclin-dependent kinase 1 (Cdk1) and casein kinase 2 (CK2). Outside S phase, integrity of the AAA-ATPase domain is required for Yta7 boundary function, as defined by correct positioning of the histone chaperone Rtt106 and the chromatin remodeling complex RSC. Conversely, in S phase, Yta7 is hyperphosphorylated, causing its release from HTA1 chromatin and productive transcription. Most importantly, abrogation of Yta7 phosphorylation results in constitutive attachment of Yta7 to HTA1 chromatin, preventing efficient transcription post-recruitment of RNA polymerase II (RNAPII). Our study identified the chromatin boundary protein Yta7 as a key regulator that links S-phase kinases with RNAPII function at cell cycle-regulated histone gene promoters.
Figures


References
-
- Belotserkovskaya R, Oh S, Bondarenko VA, Orphanides G, Studitsky VM, Reinberg D 2003. FACT facilitates transcription-dependent nucleosome alteration. Science 301: 1090–1093 - PubMed
-
- Bloom J, Cross FR 2007. Multiple levels of cyclin specificity in cell-cycle control. Nat Rev Mol Cell Biol 8: 149–160 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
