A human islet cell culture system for high-throughput screening
- PMID: 22156222
- PMCID: PMC5546101
- DOI: 10.1177/1087057111430253
A human islet cell culture system for high-throughput screening
Abstract
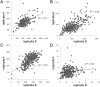
A small-molecule inducer of beta-cell proliferation in human islets represents a potential regeneration strategy for treating type 1 diabetes. However, the lack of suitable human beta cell lines makes such a discovery a challenge. Here, we adapted an islet cell culture system to high-throughput screening to identify such small molecules. We prepared microtiter plates containing extracellular matrix from a human bladder carcinoma cell line. Dissociated human islets were seeded onto these plates, cultured for up to 7 days, and assessed for proliferation by simultaneous Ki67 and C-peptide immunofluorescence. Importantly, this environment preserved beta-cell physiological function, as measured by glucose-stimulated insulin secretion. Adenoviral overexpression of cdk-6 and cyclin D(1), known inducers of human beta cell proliferation, was used as a positive control in our assay. This induction was inhibited by cotreatment with rapamycin, an immunosuppressant often used in islet transplantation. We then performed a pilot screen of 1280 compounds, observing some phenotypic effects on cells. This high-throughput human islet cell culture method can be used to assess various aspects of beta-cell biology on a relatively large number of compounds.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

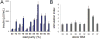

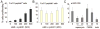

References
-
- Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet Transplantation in Seven Patients with Type 1 Diabetes Mellitus Using a Glucocorticoid-Free Immunosuppressive Regimen. N. Engl. J. Med. 2000;343:230–238. - PubMed
-
- Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, et al. International Trial of the Edmonton Protocol for Islet Transplantation. N. Engl. J. Med. 2006;355:1318–1330. - PubMed
-
- Meier JJ, Bhushan A, Butler AE, Rizza RA, Butler PC. Sustained Beta Cell Apoptosis in Patients with Long-Standing Type 1 Diabetes: Indirect Evidence for Islet Regeneration? Diabetologia. 2005;48:2221–2228. - PubMed
-
- Meier JJ, Lin JC, Butler AE, Galasso R, Martinez DS, Butler PC. Direct Evidence of Attempted Beta Cell Regeneration in an 89-Year-Old Patient with Recent-Onset Type 1 Diabetes. Diabetologia. 2006;49:1838–1844. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

