New function for the RNA helicase p68/DDX5 as a modifier of MBNL1 activity on expanded CUG repeats
- PMID: 22156369
- PMCID: PMC3326330
- DOI: 10.1093/nar/gkr1228
New function for the RNA helicase p68/DDX5 as a modifier of MBNL1 activity on expanded CUG repeats
Abstract
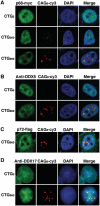
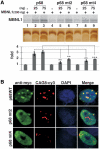
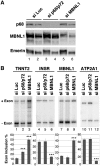
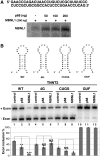
Myotonic Dystrophy type I (DM1) is caused by an abnormal expansion of CTG triplets in the 3' UTR of the dystrophia myotonica protein kinase (DMPK) gene, leading to the aggregation of the mutant transcript in nuclear RNA foci. The expanded mutant transcript promotes the sequestration of the MBNL1 splicing factor, resulting in the misregulation of a subset of alternative splicing events. In this study, we identify the DEAD-box RNA helicase p68 (DDX5) in complexes assembled onto in vitro-transcribed CUG repeats. We showed that p68 colocalized with RNA foci in cells expressing the 3'UTR of the DMPK gene containing expanded CTG repeats. We found that p68 increased MBNL1 binding onto pathological repeats and the stem-loop structure regulatory element within the cardiac Troponin T (TNNT2) pre-mRNA, splicing of which is misregulated in DM1. Mutations in the helicase core of p68 prevented both the stimulatory effect of the protein on MBNL1 binding and the colocalization of p68 with CUG repeats, suggesting that remodeling of RNA secondary structure by p68 facilitates MBNL1 binding. We also found that the competence of p68 for regulating TNNT2 exon 5 inclusion depended on the integrity of MBNL1 binding sites. We propose that p68 acts as a modifier of MBNL1 activity on splicing targets and pathogenic RNA.
Figures
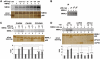
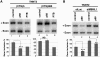
Similar articles
-
DDX6 regulates sequestered nuclear CUG-expanded DMPK-mRNA in dystrophia myotonica type 1.Nucleic Acids Res. 2014 Jun;42(11):7186-200. doi: 10.1093/nar/gku352. Epub 2014 May 3. Nucleic Acids Res. 2014. PMID: 24792155 Free PMC article.
-
Colocalization of muscleblind with RNA foci is separable from mis-regulation of alternative splicing in myotonic dystrophy.J Cell Sci. 2005 Jul 1;118(Pt 13):2923-33. doi: 10.1242/jcs.02404. Epub 2005 Jun 16. J Cell Sci. 2005. PMID: 15961406
-
Cytoplasmic CUG RNA foci are insufficient to elicit key DM1 features.PLoS One. 2008;3(12):e3968. doi: 10.1371/journal.pone.0003968. Epub 2008 Dec 18. PLoS One. 2008. PMID: 19092997 Free PMC article.
-
Dysfunction of protein homeostasis in myotonic dystrophies.Histol Histopathol. 2013 Sep;28(9):1089-98. doi: 10.14670/HH-28.1089. Epub 2013 Mar 28. Histol Histopathol. 2013. PMID: 23536431 Review.
-
Gain of RNA function in pathological cases: Focus on myotonic dystrophy.Biochimie. 2011 Nov;93(11):2006-12. doi: 10.1016/j.biochi.2011.06.028. Epub 2011 Jul 13. Biochimie. 2011. PMID: 21763392 Review.
Cited by
-
Deregulation of RNA Metabolism in Microsatellite Expansion Diseases.Adv Neurobiol. 2018;20:213-238. doi: 10.1007/978-3-319-89689-2_8. Adv Neurobiol. 2018. PMID: 29916021 Free PMC article. Review.
-
Myotonic dystrophy: approach to therapy.Curr Opin Genet Dev. 2017 Jun;44:135-140. doi: 10.1016/j.gde.2017.03.007. Epub 2017 Apr 1. Curr Opin Genet Dev. 2017. PMID: 28376341 Free PMC article. Review.
-
microRNA-mRNA Profile of Skeletal Muscle Differentiation and Relevance to Congenital Myotonic Dystrophy.Int J Mol Sci. 2021 Mar 7;22(5):2692. doi: 10.3390/ijms22052692. Int J Mol Sci. 2021. PMID: 33799993 Free PMC article.
-
Functions of DEAD box RNA helicases DDX5 and DDX17 in chromatin organization and transcriptional regulation.BMB Rep. 2018 Dec;51(12):613-622. doi: 10.5483/BMBRep.2018.51.12.234. BMB Rep. 2018. PMID: 30293550 Free PMC article. Review.
-
Myotonic dystrophy: is a narrow focus obscuring the rest of the field?Curr Opin Neurol. 2012 Oct;25(5):609-13. doi: 10.1097/WCO.0b013e328357b0d9. Curr Opin Neurol. 2012. PMID: 22892953 Free PMC article. Review.
References
-
- Harper PS. Myotonic Dystrophy. London: W. B. Saunders; 2001.
-
- Brook JD, McCurrach ME, Harley HG, Buckler AJ, Church D, Aburatani H, Hunter K, Stanton VP, Thirion JP, Hudson T, et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell. 1992;68:799–808. - PubMed
-
- Mahadevan M, Tsilfidis C, Sabourin L, Shutler G, Amemiya C, Jansen G, Neville C, Narang M, Barcelo J, O'Hoy K, et al. Myotonic dystrophy mutation: an unstable CTG repeat in the 3' untranslated region of the gene. Science. 1992;255:1253–1255. - PubMed
-
- Fu YH, Pizzuti A, Fenwick RG, Jr, King J, Rajnarayan S, Dunne PW, Dubel J, Nasser GA, Ashizawa T, de Jong P, et al. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science. 1992;255:1256–1258. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

