Conformational changes in Sindbis virus induced by decreased pH are revealed by small-angle neutron scattering
- PMID: 22156534
- PMCID: PMC3302394
- DOI: 10.1128/JVI.06569-11
Conformational changes in Sindbis virus induced by decreased pH are revealed by small-angle neutron scattering
Abstract
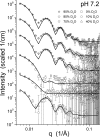
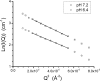
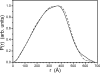
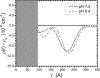
Alphaviruses, such as Sindbis virus, undergo dramatic changes in three-dimensional structure upon exposure to low pH, and such exposure can establish conditions allowing fusion of the virus membrane with a cell plasma membrane upon return to neutral pH. While exposure to low pH is not required for entry of Sindbis virus into vertebrate or invertebrate cells, the conformational changes occurring at low pH may mimic those occurring upon virus-receptor interaction. Here, we employed small-angle neutron scattering with contrast variation to probe how the structure of a mammalian-grown Sindbis virus responds to moderately acidic pH. Several changes took place throughout the virion structure when the pH decreased from 7.2 to 6.4. Specifically, the RNA in the virion core underwent a conformational change. Additionally, the protein was redistributed. A significant amount of protein moved from the layer containing the lipid bilayer to the exterior of the virion. The results improve our understanding of the pH-driven alteration of Sindbis virus structure.
Figures

Similar articles
-
Conformational changes in Sindbis virions resulting from exposure to low pH and interactions with cells suggest that cell penetration may occur at the cell surface in the absence of membrane fusion.Virology. 2004 Jul 1;324(2):373-86. doi: 10.1016/j.virol.2004.03.046. Virology. 2004. PMID: 15207623
-
The structure of Sindbis virus produced from vertebrate and invertebrate hosts as determined by small-angle neutron scattering.J Virol. 2010 May;84(10):5270-6. doi: 10.1128/JVI.00044-10. Epub 2010 Mar 10. J Virol. 2010. PMID: 20219936 Free PMC article.
-
Conformational alteration of Sindbis virion glycoproteins induced by heat, reducing agents, or low pH.J Virol. 1992 Jun;66(6):3504-13. doi: 10.1128/JVI.66.6.3504-3513.1992. J Virol. 1992. PMID: 1374808 Free PMC article.
-
Sindbis virus as a model for studies of conformational changes in a metastable virus and the role of conformational changes in in vitro antibody neutralisation.Rev Med Virol. 2009 Sep;19(5):257-72. doi: 10.1002/rmv.619. Rev Med Virol. 2009. PMID: 19475572 Review.
-
Molecular Modeling for Better Understanding of Cucumovirus Pathology.Adv Virus Res. 2018;102:59-88. doi: 10.1016/bs.aivir.2018.06.002. Epub 2018 Jul 26. Adv Virus Res. 2018. PMID: 30266176 Review.
Cited by
-
Nipah virion entry kinetics, composition, and conformational changes determined by enzymatic virus-like particles and new flow virometry tools.J Virol. 2014 Dec;88(24):14197-206. doi: 10.1128/JVI.01632-14. Epub 2014 Oct 1. J Virol. 2014. PMID: 25275126 Free PMC article.
-
Absorption of the [bmim][Cl] Ionic Liquid in DMPC Lipid Bilayers across Their Gel, Ripple, and Fluid Phases.J Phys Chem B. 2022 May 5;126(17):3309-3318. doi: 10.1021/acs.jpcb.2c00710. Epub 2022 Apr 26. J Phys Chem B. 2022. PMID: 35472281 Free PMC article.
-
Full inactivation of alphaviruses in single particle and crystallized forms.J Virol Methods. 2016 Oct;236:237-244. doi: 10.1016/j.jviromet.2016.07.020. Epub 2016 Jul 25. J Virol Methods. 2016. PMID: 27465218 Free PMC article.
-
Development of a stable virus-like particle vaccine formulation against Chikungunya virus and investigation of the effects of polyanions.J Pharm Sci. 2013 Dec;102(12):4305-14. doi: 10.1002/jps.23749. Epub 2013 Oct 15. J Pharm Sci. 2013. PMID: 24129946 Free PMC article.
-
Serial femtosecond X-ray diffraction of enveloped virus microcrystals.Struct Dyn. 2015 Aug 20;2(4):041720. doi: 10.1063/1.4929410. eCollection 2015 Jul. Struct Dyn. 2015. PMID: 26798819 Free PMC article.
References
-
- Aramayo R, et al. 2005. Divalent ion-dependent swelling of tomato bushy stunt virus: a multi-approach study. Biochim. Biophys. Acta 1724:345–354 - PubMed
-
- Chauvin C, Witz J, Jacrot B. 1978. Structure of tomato bushy stunt virus—model for protein-RNA interaction. J. Mol. Biol. 124:641–651 - PubMed
-
- Cusack S, Ruigrok RWH, Krygsman PCJ, Mellema JE. 1985. Structure and composition of influenza-virus—a small-angle neutron-scattering study. J. Mol. Biol. 186:565–582 - PubMed
-
- Dalrymple JM, Schlesinger S, Russell PK. 1976. Antigenic characterization of two sindbis envelope glycoproteins separated by isoelectric focusing. Virology 69:93–103 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

