Variants in ASB10 are associated with open-angle glaucoma
- PMID: 22156576
- PMCID: PMC3284122
- DOI: 10.1093/hmg/ddr572
Variants in ASB10 are associated with open-angle glaucoma
Abstract
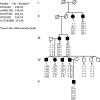
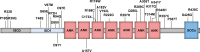
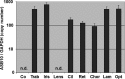
The molecular events responsible for obstruction of aqueous humor outflow and the loss of retinal ganglion cells in glaucoma, one of the main causes of blindness worldwide, remain poorly understood. We identified a synonymous variant, c.765C>T (Thr255Thr), in ankyrin repeats and suppressor of cytokine signaling box-containing protein 10 (ASB10) in a large family with primary open angle glaucoma (POAG) mapping to the GLC1F locus. This variant affects an exon splice enhancer site and alters mRNA splicing in lymphoblasts of affected family members. Systematic sequence analysis in two POAG patient groups (195 US and 977 German) and their respective controls (85 and 376) lead to the identification of 26 amino acid changes in 70 patients (70 of 1172; 6.0%) compared with 9 in 13 controls (13 of 461; 2.8%; P = 0.008). Molecular modeling suggests that these missense variants change ASB10 net charge or destabilize ankyrin repeats. ASB10 mRNA and protein were found to be strongly expressed in trabecular meshwork, retinal ganglion cells and ciliary body. Silencing of ASB10 transcripts in perfused anterior segment organ culture reduced outflow facility by ∼50% compared with control-infected anterior segments (P = 0.02). In conclusion, genetic and molecular analyses provide evidence for ASB10 as a glaucoma-causing gene.
Figures







References
-
- Quigley H.A. Glaucoma. Lancet. 2011;377:1367–1377. doi:10.1016/S0140-6736(10)61423-7. - DOI - PubMed
-
- Drance S.M., Sweeney V.P., Morgan R.W., Feldman F. Studies of factors involved in the production of low tension glaucoma. Arch. Ophthalmol. 1973;89:457–465. doi:10.1001/archopht.1973.01000040459003. - DOI - PubMed
-
- Goldwyn R., Waltman S.R., Becker B. Primary open-angle glaucoma in adolescents and young adults. Arch. Ophthalmol. 1970;84:579–582. doi:10.1001/archopht.1970.00990040581004. - DOI - PubMed
-
- Allingham R.R., Liu Y., Rhee D.J. The genetics of primary open-angle glaucoma: a review. Exp. Eye Res. 2009;88:837–844. doi:10.1016/j.exer.2008.11.003. - DOI - PMC - PubMed
-
- Fingert J.H., Robin A.L., Stone J.L., Roos B.R., Davis L.K., Scheetz T.E., Bennett S.R., Wassink T.H., Kwon Y.H., Alward W.L., et al. Copy number variations on chromosome 12q14 in patients with normal tension glaucoma. Hum. Mol. Genet. 2011;20:2482–2494. doi:10.1093/hmg/ddr123. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EY010572/EY/NEI NIH HHS/United States
- R01 EY011650/EY/NEI NIH HHS/United States
- R01 EY003279/EY/NEI NIH HHS/United States
- R01 EY019643/EY/NEI NIH HHS/United States
- EY008247/EY/NEI NIH HHS/United States
- EY011650/EY/NEI NIH HHS/United States
- EY010555/EY/NEI NIH HHS/United States
- R01 MH059490/MH/NIMH NIH HHS/United States
- R37 MH059490/MH/NIMH NIH HHS/United States
- UL1 R024140/PHS HHS/United States
- R01 EY008247/EY/NEI NIH HHS/United States
- MH059490/MH/NIMH NIH HHS/United States
- EY003279/EY/NEI NIH HHS/United States
- R01 EY010555/EY/NEI NIH HHS/United States
- P30 EY010572/EY/NEI NIH HHS/United States
- EY019643/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

