Loss of B7-H1 expression by recipient parenchymal cells leads to expansion of infiltrating donor CD8+ T cells and persistence of graft-versus-host disease
- PMID: 22156590
- PMCID: PMC3891913
- DOI: 10.4049/jimmunol.1102630
Loss of B7-H1 expression by recipient parenchymal cells leads to expansion of infiltrating donor CD8+ T cells and persistence of graft-versus-host disease
Abstract
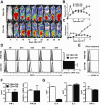
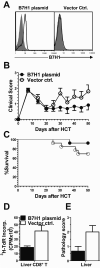
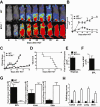
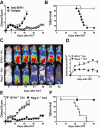
Previous experimental studies have shown that acute graft-versus-host disease (GVHD) is associated with two waves of donor CD8(+) T cell expansion. In the current studies, we used in vivo bioluminescent imaging, in vivo BrdU labeling, and three different experimental GVHD systems to show that B7-H1 expression by recipient parenchymal cells controls the second wave of alloreactive donor CD8(+) T cell expansion and the associated second phase of GVHD. Loss of B7-H1 expression by parenchymal cells during the course of GVHD was associated with persistent proliferation of donor CD8(+) T cells in GVHD target tissues and continued tissue injury, whereas persistent expression of B7-H1 expression by parenchymal cells led to reduced proliferation of donor CD8(+) T cells in GVHD target tissues and resolution of GVHD. These studies demonstrate that parenchymal cell expression of B7-H1 is required for tolerizing infiltrating T cells and preventing the persistence of GVHD. Our results suggest that therapies designed to preserve or restore expression of B7-H1 expression by parenchymal tissues in the recipient could prevent or ameliorate GVHD in humans.
Figures

Similar articles
-
Identification of stem cell transcriptional programs normally expressed in embryonic and neural stem cells in alloreactive CD8+ T cells mediating graft-versus-host disease.Biol Blood Marrow Transplant. 2010 Jun;16(6):751-71. doi: 10.1016/j.bbmt.2010.01.012. Epub 2010 Jan 29. Biol Blood Marrow Transplant. 2010. PMID: 20116439 Free PMC article.
-
PD-L1 blockade effectively restores strong graft-versus-leukemia effects without graft-versus-host disease after delayed adoptive transfer of T-cell receptor gene-engineered allogeneic CD8+ T cells.Blood. 2011 Jan 20;117(3):1030-41. doi: 10.1182/blood-2010-04-283119. Epub 2010 Nov 9. Blood. 2011. PMID: 21063028 Free PMC article.
-
PD-L1 interacts with CD80 to regulate graft-versus-leukemia activity of donor CD8+ T cells.J Clin Invest. 2017 May 1;127(5):1960-1977. doi: 10.1172/JCI91138. Epub 2017 Apr 17. J Clin Invest. 2017. PMID: 28414296 Free PMC article.
-
Unusual patterns of alloimmunity evoked by allogeneic liver parenchymal cells.Immunol Rev. 2000 Apr;174:260-79. doi: 10.1034/j.1600-0528.2002.017409.x. Immunol Rev. 2000. PMID: 10807522 Review.
-
Cell migration, chimerism, and graft acceptance.Lancet. 1992 Jun 27;339(8809):1579-82. doi: 10.1016/0140-6736(92)91840-5. Lancet. 1992. PMID: 1351558 Free PMC article. Review. No abstract available.
Cited by
-
Host programmed death ligand 1 is dominant over programmed death ligand 2 expression in regulating graft-versus-host disease lethality.Blood. 2013 Oct 24;122(17):3062-73. doi: 10.1182/blood-2013-05-500801. Epub 2013 Sep 12. Blood. 2013. PMID: 24030385 Free PMC article.
-
Tolerizing CTL by Sustained Hepatic PD-L1 Expression Provides a New Therapy Approach in Mouse Sepsis.Theranostics. 2019 Mar 16;9(7):2003-2016. doi: 10.7150/thno.28057. eCollection 2019. Theranostics. 2019. PMID: 31037153 Free PMC article.
-
Acute Graft-versus-Host Disease - Biologic Process, Prevention, and Therapy.N Engl J Med. 2017 Nov 30;377(22):2167-2179. doi: 10.1056/NEJMra1609337. N Engl J Med. 2017. PMID: 29171820 Free PMC article. Review. No abstract available.
-
Anti-CD4 monoclonal antibody prevents chronic graft-versus-host disease in mice by inducing immune tolerance of CD8+ T cells and alleviating thymus injury.Front Immunol. 2024 Dec 24;15:1460687. doi: 10.3389/fimmu.2024.1460687. eCollection 2024. Front Immunol. 2024. PMID: 39776911 Free PMC article.
-
Immune checkpoint blockade and CAR-T cell therapy in hematologic malignancies.J Hematol Oncol. 2019 Jun 11;12(1):59. doi: 10.1186/s13045-019-0746-1. J Hematol Oncol. 2019. PMID: 31186046 Free PMC article. Review.
References
-
- Zhang C, Lou J, Li N, Todorov I, Lin CL, Cao YA, Contag CH, Kandeel F, Forman S, Zeng D. Donor CD8+ T cells mediate graft-versus-leukemia activity without clinical signs of graft-versus-host disease in recipients conditioned with anti-CD3 monoclonal antibody. J Immunol. 2007;178:838–850. - PubMed
-
- Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Alloreactive memory T cells are responsible for the persistence of graft-versus-host disease. J Immunol. 2005;174:3051–3058. - PubMed
-
- Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat Med. 2005;11:1299–1305. - PubMed
-
- Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. International immunology. 1996;8:765–772. - PubMed
-
- Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

