Tracking monotonically advancing boundaries in image sequences using graph cuts and recursive kernel shape priors
- PMID: 22156978
- PMCID: PMC5510452
- DOI: 10.1109/TMI.2011.2178122
Tracking monotonically advancing boundaries in image sequences using graph cuts and recursive kernel shape priors
Abstract
We introduce a probabilistic computer vision technique to track monotonically advancing boundaries of objects within image sequences. Our method incorporates a novel technique for including statistical prior shape information into graph-cut based segmentation, with the aid of a majorization-minimization algorithm. Extension of segmentation from single images to image sequences then follows naturally using sequential Bayesian estimation. Our methodology is applied to two unrelated sets of real biomedical imaging data, and a set of synthetic images. Our results are shown to be superior to manual segmentation.
Figures





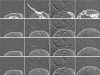



Similar articles
-
Segmentation of abdomen MR images using kernel graph cuts with shape priors.Biomed Eng Online. 2013 Dec 3;12:124. doi: 10.1186/1475-925X-12-124. Biomed Eng Online. 2013. PMID: 24295198 Free PMC article.
-
Orientation histograms as shape priors for left ventricle segmentation using graph cuts.Med Image Comput Comput Assist Interv. 2011;14(Pt 3):420-7. doi: 10.1007/978-3-642-23626-6_52. Med Image Comput Comput Assist Interv. 2011. PMID: 22003727
-
Multiregion image segmentation by parametric kernel graph cuts.IEEE Trans Image Process. 2011 Feb;20(2):545-57. doi: 10.1109/TIP.2010.2066982. Epub 2010 Aug 16. IEEE Trans Image Process. 2011. PMID: 20716502
-
A Survey of Graph Cuts/Graph Search Based Medical Image Segmentation.IEEE Rev Biomed Eng. 2018;11:112-124. doi: 10.1109/RBME.2018.2798701. Epub 2018 Jan 26. IEEE Rev Biomed Eng. 2018. PMID: 29994356 Review.
-
Rough sets and near sets in medical imaging: a review.IEEE Trans Inf Technol Biomed. 2009 Nov;13(6):955-68. doi: 10.1109/TITB.2009.2017017. Epub 2009 Mar 16. IEEE Trans Inf Technol Biomed. 2009. PMID: 19304490 Review.
Cited by
-
Heterogeneous incidence and propagation of spreading depolarizations.J Cereb Blood Flow Metab. 2017 May;37(5):1748-1762. doi: 10.1177/0271678X16659496. Epub 2016 Jan 1. J Cereb Blood Flow Metab. 2017. PMID: 27562866 Free PMC article.
-
Iterative graph cuts for image segmentation with a nonlinear statistical shape prior.J Math Imaging Vis. 2014 Mar 1;49(1):87-97. doi: 10.1007/s10851-013-0440-9. J Math Imaging Vis. 2014. PMID: 24678141 Free PMC article.
References
-
- Fast V, Kléber A. Role of wavefront curvature in propagation of cardiac impulse. Cardiovascular research. 1997;33(2):258. - PubMed
-
- Hogea C, Murray B, Sethian J. Simulating complex tumor dynamics from avascular to vascular growth using a general level-set method. journal of mathematical biology. 2006;53(1):86–134. - PubMed
-
- Cornell-Bell A, Finkbeiner S, Cooper M, Smith S. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247(4941):470. - PubMed
-
- Leao A. Spreading depression of activity in the cerebral cortex. journal of Neurophysiology. 1944;7(6):359. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

