Regulation of glycolytic enzyme phosphoglycerate mutase-1 by Sirt1 protein-mediated deacetylation
- PMID: 22157007
- PMCID: PMC3281715
- DOI: 10.1074/jbc.M111.317404
Regulation of glycolytic enzyme phosphoglycerate mutase-1 by Sirt1 protein-mediated deacetylation
Abstract
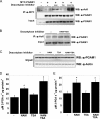
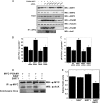
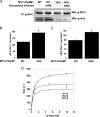
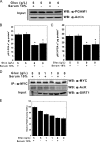
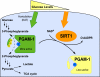
Emerging proteomic evidence suggests that acetylation of metabolic enzymes is a prevalent post-translational modification. In a few recent reports, acetylation down-regulated activity of specific enzymes in fatty acid oxidation, urea cycle, electron transport, and anti-oxidant pathways. Here, we reveal that the glycolytic enzyme phosphoglycerate mutase-1 (PGAM1) is negatively regulated by Sirt1, a member of the NAD(+)-dependent protein deacetylases. Acetylated PGAM1 displays enhanced activity, although Sirt1-mediated deacetylation reduces activity. Acetylation sites mapped to the C-terminal "cap," a region previously known to affect catalytic efficiency. Overexpression of a constitutively active variant (acetylated mimic) of PGAM1 stimulated flux through glycolysis. Under glucose restriction, Sirt1 levels dramatically increased, leading to PGAM1 deacetylation and attenuated activity. Previously, Sirt1 has been implicated in the adaptation from glucose to fat burning. This study (i) demonstrates that protein acetylation can stimulate metabolic enzymes, (ii) provides biochemical evidence that glycolysis is modulated by reversible acetylation, and (iii) demonstrates that PGAM1 deacetylation and activity are directly controlled by Sirt1.
Figures

References
-
- Hirschey M. D., Shimazu T., Goetzman E., Jing E., Schwer B., Lombard D. B., Grueter C. A., Harris C., Biddinger S., Ilkayeva O. R., Stevens R. D., Li Y., Saha A. K., Ruderman N. B., Bain J. R., Newgard C. B., Farese R. V., Jr., Alt F. W., Kahn C. R., Verdin E. (2010) SIRT3 regulates mitochondrial fatty acid oxidation by reversible enzyme deacetylation. Nature 464, 121–125 - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

