Evolution of therapies for chronic myelogenous leukemia
- PMID: 22157290
- PMCID: PMC3243359
- DOI: 10.1097/PPO.0b013e31823dec8d
Evolution of therapies for chronic myelogenous leukemia
Abstract
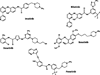
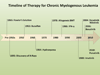
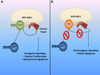
The clinical outcome for patients with chronic myelogenous leukemia (CML) has changed dramatically in the past 15 years. This has been due to the development of tyrosine kinase inhibitors (TKIs), compounds that inhibit the activity of the oncogenic BCR-ABL1 protein. Imatinib was the first TKI developed for CML, and it led to high rates of complete cytogenetic responses and improved survival for patients with this disease. However, approximately 35% of patients in chronic phase treated with imatinib will develop resistance or intolerance to this drug. The recognition of the problem of imatinib failure led to the design of second-generation TKI (dasatinib, nilotinib, and bosutinib). These drugs are highly active in the scenario of imatinib resistance or intolerance. More recently, both nilotinib and dasatinib were approved for frontline use in patients with chronic phase CML. Ponatinib represents the last generation of TKI, and this drug has been developed with the aim of targeting a specific BCR-ABL1 mutation (T315I), which arises in the setting of prolonged TKI therapy and leads to resistance to all commercially available TKI. Parallel to the development of specific drugs for treating CML, major advances were made in the field of disease monitoring and standardization of response criteria. In this review, we summarize how therapy with TKI for CML has evolved during the last decade.
Figures
References
-
- Druker BJ, Guilhot F, O'Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–2417. - PubMed
-
- Goldman JM. Chronic myeloid leukemia: a historical perspective. Seminars in hematology. 2010;47(4):302–311. - PubMed
-
- Galton DA. Myleran in chronic myeloid leukaemia; results of treatment. Lancet. 1953;264(6753):208–213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

