ErbB2 and ErbB3 regulate recovery from dextran sulfate sodium-induced colitis by promoting mouse colon epithelial cell survival
- PMID: 22157714
- PMCID: PMC3289719
- DOI: 10.1038/labinvest.2011.192
ErbB2 and ErbB3 regulate recovery from dextran sulfate sodium-induced colitis by promoting mouse colon epithelial cell survival
Abstract
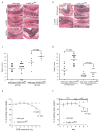
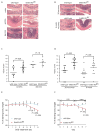
ErbB2 and ErbB3 receptor tyrosine kinases are key regulators of proliferation, migration, differentiation and cell survival; however, their roles in gastrointestinal biology remain poorly defined. We hypothesized that ErbB2 and ErbB3 promote colon epithelial cell survival in the context of the wound-healing response following colitis. In this study, mice bearing intestinal epithelial-specific deletion of ErbB2 or ErbB3 were treated with dextran sulfate sodium (DSS). Colon sections were examined for injury, cytokine expression, epithelial cell proliferation and apoptosis. Deletion of epithelial ErbB2 did not affect the extent of intestinal injury in response to DSS, whereas deletion of ErbB3 slightly increased injury. However, the roles of both receptors were more apparent during recovery from DSS colitis, in which ErbB2 or ErbB3 epithelial deletion resulted in greater inflammation and crypt damage during the early reparative period. Moreover, loss of ErbB3 prevented normal epithelial regeneration in the long term, with damage persisting for at least 6 weeks following a single round of DSS. Delayed recovery in mice with epithelial deletion of ErbB2 or ErbB3 was associated with increased colonic expression of tumor necrosis factor alpha and increased epithelial apoptosis. Furthermore, epithelial ErbB3 deletion increased apoptosis at baseline and during DSS injury. Additionally, epithelial cell hyperproliferation during recovery was exacerbated by deletion of either ErbB2 or ErbB3. These results suggest that ErbB2 and ErbB3 have important cytoprotective and reparative roles in the colonic epithelium following injury, by promoting colon epithelial cell survival.
Conflict of interest statement
Figures



Similar articles
-
Myeloid Cell-Derived IL-1 Signaling Damps Neuregulin-1 from Fibroblasts to Suppress Colitis-Induced Early Repair of the Intestinal Epithelium.Int J Mol Sci. 2024 Apr 18;25(8):4469. doi: 10.3390/ijms25084469. Int J Mol Sci. 2024. PMID: 38674054 Free PMC article.
-
Krüppel-like factor 5 protects against dextran sulfate sodium-induced colonic injury in mice by promoting epithelial repair.Gastroenterology. 2011 Feb;140(2):540-549.e2. doi: 10.1053/j.gastro.2010.10.061. Epub 2010 Nov 12. Gastroenterology. 2011. PMID: 21078320 Free PMC article.
-
Mucosa repair mechanisms of Tong-Xie-Yao-Fang mediated by CRH-R2 in murine, dextran sulfate sodium-induced colitis.World J Gastroenterol. 2018 Apr 28;24(16):1766-1778. doi: 10.3748/wjg.v24.i16.1766. World J Gastroenterol. 2018. PMID: 29713130 Free PMC article.
-
EGFR in Tumor-Associated Myeloid Cells Promotes Development of Colorectal Cancer in Mice and Associates With Outcomes of Patients.Gastroenterology. 2017 Jul;153(1):178-190.e10. doi: 10.1053/j.gastro.2017.03.053. Epub 2017 Apr 9. Gastroenterology. 2017. PMID: 28400195 Free PMC article.
-
Deletion of intestinal epithelial cell STAT3 promotes T-lymphocyte STAT3 activation and chronic colitis following acute dextran sodium sulfate injury in mice.Inflamm Bowel Dis. 2013 Mar;19(3):512-25. doi: 10.1097/MIB.0b013e31828028ad. Inflamm Bowel Dis. 2013. PMID: 23429443 Free PMC article.
Cited by
-
Identification of Potential Core Genes for the Rupture of Intracranial Aneurysms by a Bioinformatics Analysis.Front Genet. 2022 Mar 30;13:875007. doi: 10.3389/fgene.2022.875007. eCollection 2022. Front Genet. 2022. PMID: 35432454 Free PMC article.
-
Loss of ADAM17-Mediated Tumor Necrosis Factor Alpha Signaling in Intestinal Cells Attenuates Mucosal Atrophy in a Mouse Model of Parenteral Nutrition.Mol Cell Biol. 2015 Nov;35(21):3604-21. doi: 10.1128/MCB.00143-15. Epub 2015 Aug 17. Mol Cell Biol. 2015. PMID: 26283731 Free PMC article.
-
Huangqin-tang ameliorates dextran sodium sulphate-induced colitis by regulating intestinal epithelial cell homeostasis, inflammation and immune response.Sci Rep. 2016 Dec 16;6:39299. doi: 10.1038/srep39299. Sci Rep. 2016. PMID: 27982094 Free PMC article.
-
ErbB receptors and their growth factor ligands in pediatric intestinal inflammation.Pediatr Res. 2014 Jan;75(1-2):127-32. doi: 10.1038/pr.2013.210. Epub 2013 Nov 6. Pediatr Res. 2014. PMID: 24402051 Free PMC article. Review.
-
TNF Receptor 1 Promotes Early-Life Immunity and Protects against Colitis in Mice.Cell Rep. 2020 Oct 20;33(3):108275. doi: 10.1016/j.celrep.2020.108275. Cell Rep. 2020. PMID: 33086075 Free PMC article.
References
-
- Carpenter GCS. EGF: receptor interactions and the stimulation of cell growth. In: Lefkowitz R, editor. Receptors and recognition, Series B. Vol. 13. London: Chapman and Hall; 1981. pp. 43–66.
-
- Polk DB. Epidermal growth factor receptor-stimulated intestinal epithelial cell migration requires phospholipase C activity. Gastroenterology. 1998 Mar;114(3):493–502. - PubMed
-
- Procaccino F, Reinshagen M, Hoffmann P, et al. Protective effect of epidermal growth factor in an experimental model of colitis in rats. Gastroenterology. 1994 Jul;107(1):12–7. - PubMed
-
- Sinha A, Nightingale J, West KP, et al. Epidermal growth factor enemas with oral mesalamine for mild-to-moderate left-sided ulcerative colitis or proctitis. N Engl J Med. 2003 Jul 24;349(4):350–7. - PubMed
-
- Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009 Jul;9(7):463–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

