BRCA2 protein deficiency exaggerates doxorubicin-induced cardiomyocyte apoptosis and cardiac failure
- PMID: 22157755
- PMCID: PMC3325595
- DOI: 10.1074/jbc.M111.292664
BRCA2 protein deficiency exaggerates doxorubicin-induced cardiomyocyte apoptosis and cardiac failure
Abstract
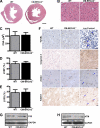
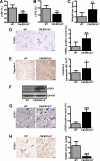
The tumor suppressor breast cancer susceptibility gene 2 (BRCA2) plays an important role in the repair of DNA damage, and loss of BRCA2 predisposes carriers to breast and ovarian cancers. Doxorubicin (DOX) remains the cornerstone of chemotherapy in such individuals. However, it is often associated with cardiac failure, which once manifests carries a poor prognosis. Because BRCA2 regulates genome-wide stability and facilitates DNA damage repair, we hypothesized that loss of BRCA2 may increase susceptibility to DOX-induced cardiac failure. To this aim, we generated cardiomyocyte-specific BRCA2 knock-out (CM-BRCA2(-/-)) mice using the Cre-loxP technology and evaluated their basal and post-DOX treatment phenotypes. Although CM-BRCA2(-/-) mice exhibited no basal cardiac phenotype, DOX treatment resulted in markedly greater cardiac dysfunction and mortality in CM-BRCA2(-/-) mice compared with control mice. Apoptosis in left ventricular (LV) sections from CM-BRCA2(-/-) mice compared with that in corresponding sections from wild-type (WT) littermate controls was also significantly enhanced after DOX treatment. Microscopic examination of LV sections from DOX-treated CM-BRCA2(-/-) mice revealed a greater number of DNA double-stranded breaks and the absence of RAD51 focus formation, an essential marker of double-stranded break repair. The levels of p53 and the p53-related proapoptotic proteins p53-up-regulated modulator of apoptosis (PUMA) and Bax were significantly increased in samples from CM-BRCA2(-/-) mice. This corresponded with increased Bax to Bcl-2 ratios and elevated cytochrome c release in the LV sections of DOX-treated CM-BRCA2(-/-) mice. Taken together, these data suggest a critical and previously unrecognized role of BRCA2 as a gatekeeper of DOX-induced cardiomyocyte apoptosis and susceptibility to overt cardiac failure. Pharmacogenomic studies evaluating cardiac function in BRCA2 mutation carriers treated with doxorubicin are encouraged.
Figures


References
-
- Rahman N., Stratton M. R. (1998) The genetics of breast cancer susceptibility. Annu. Rev. Genet. 32, 95–121 - PubMed
-
- Kinzler K. W., Vogelstein B. (1997) Cancer-susceptibility genes. Gatekeepers and caretakers. Nature 386, 761–763 - PubMed
-
- Connor F., Bertwistle D., Mee P. J., Ross G. M., Swift S., Grigorieva E., Tybulewicz V. L., Ashworth A. (1997) Tumorigenesis and a DNA repair defect in mice with a truncating Brca2 mutation. Nat. Genet. 17, 423–430 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

