Five dysfunctional telomeres predict onset of senescence in human cells
- PMID: 22157895
- PMCID: PMC3246253
- DOI: 10.1038/embor.2011.227
Five dysfunctional telomeres predict onset of senescence in human cells
Abstract
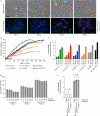
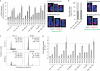
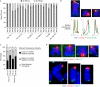
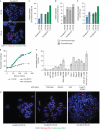
Replicative senescence is accompanied by a telomere-specific DNA damage response (DDR). We found that DDR+ telomeres occur spontaneously in early-passage normal human cells and increase in number with increasing cumulative cell divisions. DDR+ telomeres at replicative senescence retain TRF2 and RAP1 proteins, are not associated with end-to-end fusions and mostly result from strand-independent, postreplicative dysfunction. On the basis of the calculated number of DDR+ telomeres in G1-phase cells just before senescence and after bypassing senescence by inactivation of wild-type p53 function, we conclude that the accrual of five telomeres in G1 that are DDR+ but nonfusogenic is associated with p53-dependent senescence.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Comment in
-
Telomere flip-flop: an unfolding passage to senescence.EMBO Rep. 2011 Dec 23;13(1):5-6. doi: 10.1038/embor.2011.237. EMBO Rep. 2011. PMID: 22157893 Free PMC article. No abstract available.
References
-
- Bailey SM, Cornforth MN, Kurimasa A, Chen DJ, Goodwin EH (2001) Strand-specific postreplicative processing of mammalian telomeres. Science 293: 2462–2465 - PubMed
-
- Bryan TM, Reddel RR (1994) SV40-induced immortalization of human cells. Crit Rev Oncog 5: 331–357 - PubMed
-
- Cesare AJ, Kaul Z, Cohen SB, Napier CE, Pickett HA, Neumann AA, Reddel RR (2009) Spontaneous occurrence of telomeric DNA damage response in the absence of chromosome fusions. Nat Struct Mol Biol 16: 1244–1251 - PubMed
-
- Colgin LM, Reddel RR (1999) Telomere maintenance mechanisms and cellular immortalization. Curr Opin Genet Dev 9: 97–103 - PubMed
-
- Crabbe L, Verdun RE, Haggblom CI, Karlseder J (2004) Defective telomere lagging strand synthesis in cells lacking WRN helicase activity. Science 306: 1951–1953 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

