The basis for monitoring strategies in clinical guidelines: a case study of prostate-specific antigen for monitoring in prostate cancer
- PMID: 22158408
- PMCID: PMC3273504
- DOI: 10.1503/cmaj.110600
The basis for monitoring strategies in clinical guidelines: a case study of prostate-specific antigen for monitoring in prostate cancer
Abstract
Background: The volume of published literature on the evaluation and use of tests for monitoring purposes is sparse. Our aim was to determine the extent to which recommendations for monitoring prostate-specific antigen to detect recurrent prostate cancer consider key factors that should inform rule-based strategies for monitoring.
Methods: We reviewed the recommendations made in clinical guidelines for the repeated measurement of prostate-specific antigen in men who have received primary treatment for localized prostate cancer. We assessed the guidelines using the Appraisal of Guidelines for Research and Evaluation Framework.
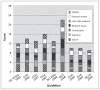
Results: We identified guidelines and statements of best practice from nine organizations. We saw considerable inconsistency in recommendations for testing for prostate-specific antigen as a form of monitoring. Recommendations on when to test appeared to be almost exclusively determined using standard follow-up schedules rather than any scientific basis. Recommendations on when to take action were primarily based on consensus statements or retrospective case series. Eight of the nine guidelines acknowledged the potential presence of measurement variability, but they did not attempt to account for the effect of such variability on the interpretation of the results of tests for prostate-specific antigen. Many recommendations were made with few or no supporting references; however, a variety of papers were cited across guidelines. Of 48 papers cited, 29.1% (14/48) were reviews; the remaining 70.8% (34/48) of papers cited were primary studies.
Interpretation: A systematic approach to the development of monitoring schedules using prostate-specific antigen in guidelines for prostate cancer is lacking, due to inadequacies in the available evidence and its use.
Figures
Comment in
-
Guidelines are too important to be left to clinical experts.CMAJ. 2012 Feb 7;184(2):159-60. doi: 10.1503/cmaj.111989. Epub 2011 Dec 19. CMAJ. 2012. PMID: 22184368 Free PMC article. No abstract available.
References
-
- Glasziou PP, Aronson JK. An introduction to monitoring therapeutic interventions in clinical practice. In: Glasziou PP, Irwig L, Aronson JK, editors. Evidence-based medical monitoring: from principles to practice. Oxford (UK): Blackwell Publishing; 2008. p. 3–14
-
- Mant D. A framework for developing and evaluating a monitoring strategy. In: Glasziou PP, Irwig L, Aronson JK, editors. Evidence-based medical monitoring: from principles to practice. Oxford (UK): Blackwell Publishing; 2008. p. 15–30
-
- Irwig L, Glasziou PP. Choosing the best monitoring tests. In: Glasziou PP, Irwig L, Aronson JK, editors. Evidence-based medical monitoring: from principles to practice. Oxford (UK): Blackwell Publishing; 2008. p. 63–74
-
- Lilja H, Ulmert D, Vickers AJ. Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nat Rev Cancer 2008;8:268–78 - PubMed
-
- Cookson MS, Aus G, Burnett AL, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol 2007;177:540–5 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
