Arabidopsis ATM and ATR kinases prevent propagation of genome damage caused by telomere dysfunction
- PMID: 22158468
- PMCID: PMC3269864
- DOI: 10.1105/tpc.111.092387
Arabidopsis ATM and ATR kinases prevent propagation of genome damage caused by telomere dysfunction
Abstract
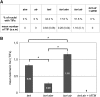
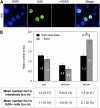
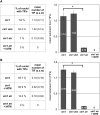
The ends of linear eukaryotic chromosomes are hidden in nucleoprotein structures called telomeres, and loss of the telomere structure causes inappropriate repair, leading to severe karyotypic and genomic instability. Although it has been shown that DNA damaging agents activate a DNA damage response (DDR), little is known about the signaling of dysfunctional plant telomeres. We show that absence of telomerase in Arabidopsis thaliana elicits an ATAXIA-TELANGIECTASIA MUTATED (ATM) and ATM AND RAD3-RELATED (ATR)-dependent DDR at telomeres, principally through ATM. By contrast, telomere dysfunction induces an ATR-dependent response in telomeric Conserved telomere maintenance component1 (Ctc1)-Suppressor of cdc thirteen (Stn1)-Telomeric pathways in association with Stn1 (CST)-complex mutants. These results uncover a new role for the CST complex in repressing the ATR-dependent DDR pathway in plant cells and show that plant cells use two different DNA damage surveillance pathways to signal telomere dysfunction. The absence of either ATM or ATR in ctc1 and stn1 mutants significantly enhances developmental and genome instability while reducing stem cell death. These data thus give a clear illustration of the action of ATM/ATR-dependent programmed cell death in maintaining genomic integrity through elimination of genetically unstable cells.
Figures




References
-
- Caryl A.P., Armstrong S.J., Jones G.H., Franklin F.C. (2000). A homologue of the yeast HOP1 gene is inactivated in the Arabidopsis meiotic mutant asy1. Chromosoma 109: 62–71 - PubMed
-
- Celli G.B., de Lange T. (2005). DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion. Nat. Cell Biol. 7: 712–718 - PubMed
-
- Charbonnel C., Gallego M.E., White C.I. (2010). Xrcc1-dependent and Ku-dependent DNA double-strand break repair kinetics in Arabidopsis plants. Plant J. 64: 280–290 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

