Coactivation of thalamic and cortical pathways induces input timing-dependent plasticity in amygdala
- PMID: 22158512
- PMCID: PMC3245819
- DOI: 10.1038/nn.2993
Coactivation of thalamic and cortical pathways induces input timing-dependent plasticity in amygdala
Abstract
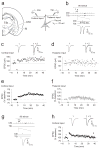
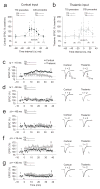
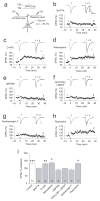
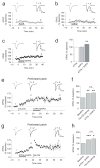
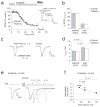
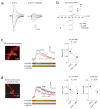
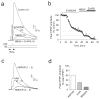
Long-term synaptic enhancements in cortical and thalamic auditory inputs to the lateral nucleus of the amygdala (LAn) mediate encoding of conditioned fear memory. It is not known, however, whether the convergent auditory conditioned stimulus (CSa) pathways interact with each other to produce changes in their synaptic function. We found that continuous paired stimulation of thalamic and cortical auditory inputs to the LAn with the interstimulus delay approximately mimicking a temporal pattern of their activation in behaving animals during auditory fear conditioning resulted in persistent potentiation of synaptic transmission in the cortico-amygdala pathway in rat brain slices. This form of input timing-dependent plasticity (ITDP) in cortical input depends on inositol 1,4,5-trisphosphate (InsP(3))-sensitive Ca(2+) release from internal stores and postsynaptic Ca(2+) influx through calcium-permeable kainate receptors during its induction. ITDP in the auditory projections to the LAn, determined by characteristics of presynaptic activity patterns, may contribute to the encoding of the complex CSa.
Figures
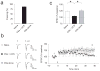
References
-
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. - PubMed
-
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. - PubMed
-
- Armony JL, Servan-Schreiber D, Romanski LM, Cohen JD, LeDoux JE. Stimulus generalization of fear responses: effects of auditory cortex lesions in a computational model and in rats. Cereb Cortex. 1997;7:157–165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

