Apoptosis-dependent externalization and involvement in apoptotic cell clearance of DmCaBP1, an endoplasmic reticulum protein of Drosophila
- PMID: 22158613
- PMCID: PMC3270968
- DOI: 10.1074/jbc.M111.277921
Apoptosis-dependent externalization and involvement in apoptotic cell clearance of DmCaBP1, an endoplasmic reticulum protein of Drosophila
Abstract
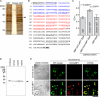
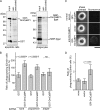
To elucidate the actions of Draper, a receptor responsible for the phagocytic clearance of apoptotic cells in Drosophila, we isolated proteins that bind to the extracellular region of Draper using affinity chromatography. One of those proteins has been identified to be an uncharacterized protein called Drosophila melanogaster calcium-binding protein 1 (DmCaBP1). This protein containing the thioredoxin-like domain resided in the endoplasmic reticulum and seemed to be expressed ubiquitously throughout the development of Drosophila. DmCaBP1 was externalized without truncation after the induction of apoptosis somewhat prior to chromatin condensation and DNA cleavage in a manner dependent on the activity of caspases. A recombinant DmCaBP1 protein bound to both apoptotic cells and a hemocyte-derived cell line expressing Draper. Forced expression of DmCaBP1 at the cell surface made non-apoptotic cells susceptible to phagocytosis. Flies deficient in DmCaBP1 expression developed normally and showed Draper-mediated pruning of larval axons, but a defect in the phagocytosis of apoptotic cells in embryos was observed. Loss of Pretaporter, a previously identified ligand for Draper, did not cause a further decrease in the level of phagocytosis in DmCaBP1-lacking embryos. These results collectively suggest that the endoplasmic reticulum protein DmCaBP1 is externalized upon the induction of apoptosis and serves as a tethering molecule to connect apoptotic cells and phagocytes for effective phagocytosis to occur.
Figures

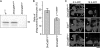

Similar articles
-
Pretaporter, a Drosophila protein serving as a ligand for Draper in the phagocytosis of apoptotic cells.EMBO J. 2009 Dec 16;28(24):3868-78. doi: 10.1038/emboj.2009.343. EMBO J. 2009. PMID: 19927123 Free PMC article.
-
Draper-mediated and phosphatidylserine-independent phagocytosis of apoptotic cells by Drosophila hemocytes/macrophages.J Biol Chem. 2004 Nov 12;279(46):48466-76. doi: 10.1074/jbc.M408597200. Epub 2004 Sep 1. J Biol Chem. 2004. PMID: 15342648
-
Integrin βν-mediated phagocytosis of apoptotic cells in Drosophila embryos.J Biol Chem. 2011 Jul 22;286(29):25770-7. doi: 10.1074/jbc.M110.204503. Epub 2011 May 18. J Biol Chem. 2011. PMID: 21592968 Free PMC article.
-
Apoptotic Cell Clearance in Development.Curr Top Dev Biol. 2015;114:297-334. doi: 10.1016/bs.ctdb.2015.07.024. Epub 2015 Sep 9. Curr Top Dev Biol. 2015. PMID: 26431572 Review.
-
Phagocyte Responses to Cell Death in Flies.Cold Spring Harb Perspect Biol. 2020 Apr 1;12(4):a036350. doi: 10.1101/cshperspect.a036350. Cold Spring Harb Perspect Biol. 2020. PMID: 31501193 Free PMC article. Review.
Cited by
-
Integrin αPS3/βν-mediated phagocytosis of apoptotic cells and bacteria in Drosophila.J Biol Chem. 2013 Apr 12;288(15):10374-80. doi: 10.1074/jbc.M113.451427. Epub 2013 Feb 20. J Biol Chem. 2013. PMID: 23426364 Free PMC article.
-
Glial Draper Rescues Aβ Toxicity in a Drosophila Model of Alzheimer's Disease.J Neurosci. 2017 Dec 6;37(49):11881-11893. doi: 10.1523/JNEUROSCI.0862-17.2017. Epub 2017 Nov 6. J Neurosci. 2017. PMID: 29109235 Free PMC article.
-
Modular Organization of Engulfment Receptors and Proximal Signaling Networks: Avenues to Reprogram Phagocytosis.Front Immunol. 2021 Apr 19;12:661974. doi: 10.3389/fimmu.2021.661974. eCollection 2021. Front Immunol. 2021. PMID: 33953723 Free PMC article. Review.
-
bfc, a novel serpent co-factor for the expression of croquemort, regulates efferocytosis in Drosophila melanogaster.PLoS Genet. 2021 Dec 3;17(12):e1009947. doi: 10.1371/journal.pgen.1009947. eCollection 2021 Dec. PLoS Genet. 2021. PMID: 34860835 Free PMC article.
-
The role of secreted proteins in efferocytosis.Front Cell Dev Biol. 2024 Jan 8;11:1332482. doi: 10.3389/fcell.2023.1332482. eCollection 2023. Front Cell Dev Biol. 2024. PMID: 38259511 Free PMC article. Review.
References
-
- Liao D. J. (2005) The scavenger cell hypothesis of apoptosis. Apoptosis redefined as a process by which a cell in living tissue is destroyed by phagocytosis. Med. Hypotheses 65, 23–28 - PubMed
-
- Nakanishi Y., Nagaosa K., Shiratsuchi A. (2011) Phagocytic removal of cells that have become unwanted. Implications for animal development and tissue homeostasis. Dev. Growth Differ. 53, 149–160 - PubMed
-
- Savill J., Fadok V. (2000) Corpse clearance defines the meaning of cell death. Nature 407, 784–788 - PubMed
-
- Lauber K., Blumenthal S. G., Waibel M., Wesselborg S. (2004) Clearance of apoptotic cells. Getting rid of the corpses. Mol. Cell 14, 277–287 - PubMed
-
- Ravichandran K. S., Lorenz U. (2007) Engulfment of apoptotic cells. Signals for a good meal. Nat. Rev. Immunol. 7, 964–974 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

