Lectin site ligation of CR3 induces conformational changes and signaling
- PMID: 22158618
- PMCID: PMC3270988
- DOI: 10.1074/jbc.M111.298307
Lectin site ligation of CR3 induces conformational changes and signaling
Abstract
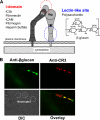
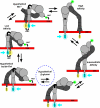
Neutrophils provide an innate immune response to tissues infected with fungal pathogens such as Candida albicans. This response is tightly regulated in part through the interaction of integrins with extracellular matrix ligands that are distributed within infected tissues. The β(2) integrin, CR3 (CD11b/CD18), is unique among integrins in containing a lectin-like domain that binds the fungal pathogen-associated molecular pattern β-glucan and serves as the dominant receptor for recognition of fungal pathogens by human granulocytes. β-Glucan, when isolated in soluble form, has been shown to be a safe and effective immune potentiator when administered therapeutically. Currently a pharmaceutical grade preparation of β-glucan is in several clinical trials with an anti-cancer indication. CR3 binding of extracellular matrix, carbohydrate, or both ligands simultaneously differentially regulates neutrophil function through a mechanism not clearly understood. Using FRET reporters, we interrogated the effects of soluble β-glucan on intracellular and extracellular CR3 structure. Although the canonical CR3 ligand fibrinogen induced full activation, β-glucan alone or in conjunction with fibrinogen stabilized an intermediate conformation with moderate headpiece extension and full cytoplasmic tail separation. A set of phosphopeptides differentially regulated by β-glucan in a CR3-dependent manner were identified using functional proteomics and found to be enriched for signaling molecules and proteins involved in transcriptional regulation, mRNA processing, and alternative splicing. These data confirm that CR3 is a signaling pattern recognition receptor for β-glucan and represent the first direct evidence of soluble β-glucan binding and affecting a signaling-competent intermediate CR3 conformation on living cells.
Figures




Similar articles
-
Function of the lectin domain of Mac-1/complement receptor type 3 (CD11b/CD18) in regulating neutrophil adhesion.J Immunol. 2002 Dec 1;169(11):6417-26. doi: 10.4049/jimmunol.169.11.6417. J Immunol. 2002. PMID: 12444150
-
Integrin Cross-Talk Regulates the Human Neutrophil Response to Fungal β-Glucan in the Context of the Extracellular Matrix: A Prominent Role for VLA3 in the Antifungal Response.J Immunol. 2017 Jan 1;198(1):318-334. doi: 10.4049/jimmunol.1502381. Epub 2016 Nov 16. J Immunol. 2017. PMID: 27852744 Free PMC article.
-
The beta-glucan-binding lectin site of mouse CR3 (CD11b/CD18) and its function in generating a primed state of the receptor that mediates cytotoxic activation in response to iC3b-opsonized target cells.J Immunol. 1999 Feb 15;162(4):2281-90. J Immunol. 1999. PMID: 9973505
-
Neutrophil Integrins and Matrix Ligands and NET Release.Front Immunol. 2016 Sep 19;7:363. doi: 10.3389/fimmu.2016.00363. eCollection 2016. Front Immunol. 2016. PMID: 27698655 Free PMC article. Review.
-
Role of the lectin domain of Mac-1/CR3 (CD11b/CD18) in regulating intercellular adhesion.Immunol Res. 2002;25(3):219-27. doi: 10.1385/IR:25:3:219. Immunol Res. 2002. PMID: 12018461 Review.
Cited by
-
Aspergillus Cell Wall Chitin Induces Anti- and Proinflammatory Cytokines in Human PBMCs via the Fc-γ Receptor/Syk/PI3K Pathway.mBio. 2016 May 31;7(3):e01823-15. doi: 10.1128/mBio.01823-15. mBio. 2016. PMID: 27247234 Free PMC article.
-
Menacing Mold: Recent Advances in Aspergillus Pathogenesis and Host Defense.J Mol Biol. 2019 Oct 4;431(21):4229-4246. doi: 10.1016/j.jmb.2019.03.027. Epub 2019 Apr 4. J Mol Biol. 2019. PMID: 30954573 Free PMC article. Review.
-
Neutrophil Extracellular Traps in Candida albicans Infection.Front Immunol. 2022 Jun 16;13:913028. doi: 10.3389/fimmu.2022.913028. eCollection 2022. Front Immunol. 2022. PMID: 35784323 Free PMC article. Review.
-
RHOA-mediated mechanical force generation through Dectin-1.J Cell Sci. 2020 Mar 2;133(5):jcs236166. doi: 10.1242/jcs.236166. J Cell Sci. 2020. PMID: 31964711 Free PMC article.
-
Potential benefit of β-glucans as adjuvant therapy in immuno-oncology: a review.Explor Target Antitumor Ther. 2021;2(2):122-138. doi: 10.37349/etat.2021.00036. Epub 2021 Apr 30. Explor Target Antitumor Ther. 2021. PMID: 36046144 Free PMC article. Review.
References
-
- Xia Y., Ross G. D. (1999) Generation of recombinant fragments of CD11b expressing the functional β-glucan-binding lectin site of CR3 (CD11b/CD18). J. Immunol. 162, 7285–7293 - PubMed
-
- Phaff H. J. (1963) Cell wall of yeasts. Annu. Rev. Microbiol. 17, 15–30 - PubMed
-
- Lavigne L. M., O'Brien X. M., Kim M., Janowski J. W., Albina J. E., Reichner J. S. (2007) Integrin engagement mediates the human polymorphonuclear leukocyte response to a fungal pathogen-associated molecular pattern. J. Immunol. 178, 7276–7282 - PubMed
-
- Harler M. B., Wakshull E., Filardo E. J., Albina J. E., Reichner J. S. (1999) Promotion of neutrophil chemotaxis through differential regulation of β1 and β2 integrins. J. Immunol. 162, 6792–6799 - PubMed
-
- Gelderman K. A., Tomlinson S., Ross G. D., Gorter A. (2004) Complement function in mAb-mediated cancer immunotherapy. Trends Immunol. 25, 158–164 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

