Hydrogen sulfide inhibits high glucose-induced matrix protein synthesis by activating AMP-activated protein kinase in renal epithelial cells
- PMID: 22158625
- PMCID: PMC3281646
- DOI: 10.1074/jbc.M111.278325
Hydrogen sulfide inhibits high glucose-induced matrix protein synthesis by activating AMP-activated protein kinase in renal epithelial cells
Abstract
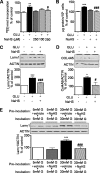
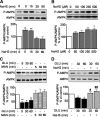
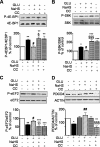
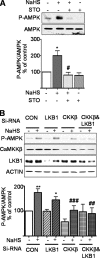
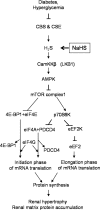
Hydrogen sulfide, a signaling gas, affects several cell functions. We hypothesized that hydrogen sulfide modulates high glucose (30 mm) stimulation of matrix protein synthesis in glomerular epithelial cells. High glucose stimulation of global protein synthesis, cellular hypertrophy, and matrix laminin and type IV collagen content was inhibited by sodium hydrosulfide (NaHS), an H(2)S donor. High glucose activation of mammalian target of rapamycin (mTOR) complex 1 (mTORC1), shown by phosphorylation of p70S6 kinase and 4E-BP1, was inhibited by NaHS. High glucose stimulated mTORC1 to promote key events in the initiation and elongation phases of mRNA translation: binding of eIF4A to eIF4G, reduction in PDCD4 expression and inhibition of its binding to eIF4A, eEF2 kinase phosphorylation, and dephosphorylation of eEF2; these events were inhibited by NaHS. The role of AMP-activated protein kinase (AMPK), an inhibitor of protein synthesis, was examined. NaHS dose-dependently stimulated AMPK phosphorylation and restored AMPK phosphorylation reduced by high glucose. Compound C, an AMPK inhibitor, abolished NaHS modulation of high glucose effect on events in mRNA translation as well as global and matrix protein synthesis. NaHS induction of AMPK phosphorylation was inhibited by siRNA for calmodulin kinase kinase β, but not LKB1, upstream kinases for AMPK; STO-609, a calmodulin kinase kinase β inhibitor, had the same effect. Renal cortical content of cystathionine β-synthase and cystathionine γ-lyase, hydrogen sulfide-generating enzymes, was significantly reduced in mice with type 1 diabetes or type 2 diabetes, coinciding with renal hypertrophy and matrix accumulation. Hydrogen sulfide is a newly identified modulator of protein synthesis in the kidney, and reduction in its generation may contribute to kidney injury in diabetes.
Figures




Similar articles
-
Tadalafil Integrates Nitric Oxide-Hydrogen Sulfide Signaling to Inhibit High Glucose-induced Matrix Protein Synthesis in Podocytes.J Biol Chem. 2015 May 8;290(19):12014-26. doi: 10.1074/jbc.M114.615377. Epub 2015 Mar 9. J Biol Chem. 2015. PMID: 25752605 Free PMC article.
-
Glycogen synthase kinase 3beta is a novel regulator of high glucose- and high insulin-induced extracellular matrix protein synthesis in renal proximal tubular epithelial cells.J Biol Chem. 2008 Nov 7;283(45):30566-75. doi: 10.1074/jbc.M801756200. Epub 2008 Aug 12. J Biol Chem. 2008. PMID: 18701453 Free PMC article.
-
A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy.Am J Physiol Renal Physiol. 2007 Feb;292(2):F617-27. doi: 10.1152/ajprenal.00278.2006. Epub 2006 Oct 3. Am J Physiol Renal Physiol. 2007. PMID: 17018841
-
LKB1 and AMP-activated protein kinase control of mTOR signalling and growth.Acta Physiol (Oxf). 2009 May;196(1):65-80. doi: 10.1111/j.1748-1716.2009.01972.x. Epub 2009 Feb 19. Acta Physiol (Oxf). 2009. PMID: 19245654 Free PMC article. Review.
-
Regulation of translation initiation by amino acids in eukaryotic cells.Prog Mol Subcell Biol. 2001;26:155-84. doi: 10.1007/978-3-642-56688-2_6. Prog Mol Subcell Biol. 2001. PMID: 11575165 Review.
Cited by
-
The Role of Hydrogen Sulfide in Renal System.Front Pharmacol. 2016 Oct 18;7:385. doi: 10.3389/fphar.2016.00385. eCollection 2016. Front Pharmacol. 2016. PMID: 27803669 Free PMC article. Review.
-
Endogenous CSE/Hydrogen Sulfide System Regulates the Effects of Glucocorticoids and Insulin on Muscle Protein Synthesis.Oxid Med Cell Longev. 2019 Apr 7;2019:9752698. doi: 10.1155/2019/9752698. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31089421 Free PMC article.
-
Hydrogen sulfide mitigates hyperglycemic remodeling via liver kinase B1-adenosine monophosphate-activated protein kinase signaling.Biochim Biophys Acta. 2014 Dec;1843(12):2816-26. doi: 10.1016/j.bbamcr.2014.08.005. Epub 2014 Aug 13. Biochim Biophys Acta. 2014. PMID: 25127936 Free PMC article.
-
Tadalafil Integrates Nitric Oxide-Hydrogen Sulfide Signaling to Inhibit High Glucose-induced Matrix Protein Synthesis in Podocytes.J Biol Chem. 2015 May 8;290(19):12014-26. doi: 10.1074/jbc.M114.615377. Epub 2015 Mar 9. J Biol Chem. 2015. PMID: 25752605 Free PMC article.
-
Hydrogen sulfide inhibits the renal fibrosis of obstructive nephropathy.Kidney Int. 2014 Jun;85(6):1318-29. doi: 10.1038/ki.2013.449. Epub 2013 Nov 27. Kidney Int. 2014. PMID: 24284510 Free PMC article.
References
-
- Scotland R. S., Madhani M., Chauhan S., Moncada S., Andresen J., Nilsson H., Hobbs A. J., Ahluwalia A. (2005) Investigation of vascular responses in endothelial nitric-oxide synthase/cyclooxygenase-1 double-knockout mice: key role for endothelium-derived hyperpolarizing factor in the regulation of blood pressure in vivo. Circulation 111, 796–803 - PubMed
-
- Zhao W., Wang R. (2002) H2S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am. J. Physiol. Heart Circ. Physiol. 283, H474–H480 - PubMed
-
- Elrod J. W., Calvert J. W., Morrison J., Doeller J. E., Kraus D. W., Tao L., Jiao X., Scalia R., Kiss L., Szabo C., Kimura H., Chow C. W., Lefer D. J. (2007) Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc. Natl. Acad. Sci. U.S.A. 104, 15560–15565 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

