Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study
- PMID: 22158731
- PMCID: PMC3260065
- DOI: 10.3945/ajcn.111.024927
Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study
Abstract
Background: Obesity is a serious chronic disease. Controlled-release phentermine/topiramate (PHEN/TPM CR), as an adjunct to lifestyle modification, has previously shown significant weight loss compared with placebo in a 56-wk study in overweight and obese subjects with ≥2 weight-related comorbidities.
Objective: This study evaluated the long-term efficacy and safety of PHEN/TPM CR in overweight and obese subjects with cardiometabolic disease.
Design: This was a placebo-controlled, double-blind, 52-wk extension study; volunteers at selected sites continued with original randomly assigned treatment [placebo, 7.5 mg phentermine/46 mg controlled-release topiramate (7.5/46), or 15 mg phentermine/92 mg controlled-release topiramate (15/92)] to complete a total of 108 wk. All subjects participated in a lifestyle-modification program.
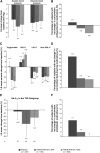
Results: Of 866 eligible subjects, 676 (78%) elected to continue in the extension. Overall, 84.0% of subjects completed the study, with similar completion rates between treatment groups. At week 108, PHEN/TPM CR was associated with significant, sustained weight loss (intent-to-treat with last observation carried forward; P < 0.0001 compared with placebo); least-squares mean percentage changes from baseline in body weight were -1.8%, -9.3%, and -10.5% for placebo, 7.5/46, and 15/92, respectively. Significantly more PHEN/TPM CR-treated subjects at each dose achieved ≥5%, ≥10%, ≥15%, and ≥20% weight loss compared with placebo (P < 0.001). PHEN/TPM CR improved cardiovascular and metabolic variables and decreased rates of incident diabetes in comparison with placebo. PHEN/TPM CR was well tolerated over 108 wk, with reduced rates of adverse events occurring between weeks 56 and 108 compared with rates between weeks 0 and 56.
Conclusion: PHEN/TPM CR in conjunction with lifestyle modification may provide a well-tolerated and effective option for the sustained treatment of obesity complicated by cardiometabolic disease. This trial was registered at clinicaltrials.gov as NCT00796367.
Figures



Comment in
-
Update in general internal medicine: evidence published in 2012.Ann Intern Med. 2013 Apr 16;158(8):615-9. doi: 10.7326/0003-4819-158-8-201304160-00101. Ann Intern Med. 2013. PMID: 23579948 No abstract available.
References
-
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA 2010;303:235–41 - PubMed
-
- Delaet D, Schauer D. Obesity in adults. Clin Evid (Online) 2011. Available from: http://clinicalevidence.bmj.com/ceweb/index.jsp (cited 10 October 2011)
-
- Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006;444:881–7 - PubMed
-
- Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA 2007;298:2028–37 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

