Phospholipase Cγ-2 and intracellular calcium are required for lipopolysaccharide-induced Toll-like receptor 4 (TLR4) endocytosis and interferon regulatory factor 3 (IRF3) activation
- PMID: 22158869
- PMCID: PMC3281733
- DOI: 10.1074/jbc.C111.328559
Phospholipase Cγ-2 and intracellular calcium are required for lipopolysaccharide-induced Toll-like receptor 4 (TLR4) endocytosis and interferon regulatory factor 3 (IRF3) activation
Abstract
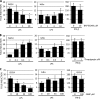
Toll-like receptor 4 (TLR4) is unique among the TLRs in its use of multiple adaptor proteins leading to activation of both the interferon regulatory factor 3 (IRF3) and nuclear factor κB (NF-κB) pathways. Previous work has demonstrated that TLR4 initiates NF-κB activation from the plasma membrane, but that subsequent TLR4 translocation to the endosomes is required for IRF3 activation. Here we have characterized several components of the signaling pathway that governs TLR4 translocation and subsequent IRF3 activation. We find that phospholipase C γ2 (PLCγ2) accounts for LPS-induced inositol 1,4,5-trisphosphate (IP(3)) production and subsequent calcium (Ca(2+)) release. Blockage of PLCγ2 function by inhibitors or knockdown of PLCγ2 expression by siRNAs in RAW 264.7 macrophages lead to reduced IRF3, but enhanced NF-κB activation. In addition, bone marrow-derived macrophages from PLCγ2-deficient mice showed impaired IRF3 phosphorylation and expression of IRF3-regulated genes after LPS stimulation. Using cell fractionation, we show that PLCγ2-IP(3)-Ca(2+) signaling cascade is required for TLR4 endocytosis following LPS stimulation. In conclusion, our results describe a novel role of the PLCγ2-IP(3)-Ca(2+) cascade in the LPS-induced innate immune response pathway where release of intracellular Ca(2+) mediates TLR4 trafficking and subsequent activation of IRF3.
Figures

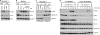

References
-
- Akira S., Takeda K., Kaisho T. (2001) Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2, 675–680 - PubMed
-
- Akira S., Uematsu S., Takeuchi O. (2006) Pathogen recognition and innate immunity. Cell 124, 783–801 - PubMed
-
- Poltorak A., He X., Smirnova I., Liu M. Y., Van Huffel C., Du X., Birdwell D., Alejos E., Silva M., Galanos C., Freudenberg M., Ricciardi-Castagnoli P., Layton B., Beutler B. (1998) Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 - PubMed
-
- Kawai T., Adachi O., Ogawa T., Takeda K., Akira S. (1999) Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 11, 115–122 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

