Interleukin (IL)-23 suppresses IL-10 in inflammatory bowel disease
- PMID: 22158873
- PMCID: PMC3271012
- DOI: 10.1074/jbc.M111.304949
Interleukin (IL)-23 suppresses IL-10 in inflammatory bowel disease
Abstract
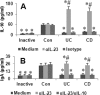
Interleukin (IL)-10 plays an important role in immune regulation in the intestine. Immune deregulation is suggested in the pathogenesis of inflammatory bowel disease (IBD). This study aims to elucidate the role of IL-23 in the suppression of IL-10 in the IBD intestinal mucosa. Surgically removed colon specimens were obtained from 16 IBD patients. The expressions of IL-10, IL-23, and IgA in the specimens were examined at the protein and gene transcriptional levels. The gene transcription of IL-10 was assessed by chromatin immunoprecipitation assay and promoter accessibility assay. The levels of IgA and IL-10 were significantly lower, whereas the levels of IL-23 were higher, in IBD specimens than in normal controls. The levels of IgA and IL-10 were negatively correlated with the infiltration of inflammatory cells in the IBD mucosa. The production of IL-10 by lamina propria mononuclear cells was lower in the IBD group than in the control group, and these levels could be enhanced by blocking IL-23. The gene transcription of IL-10 was significantly suppressed in CD4(+) T cells of IBD mucosa; this phenomenon could be replicated in vitro by adding IL-23 in the culture of polarized Th2 cells. Overexpression of IL-23 in the intestinal mucosa suppresses the production of IL-10, which weakens the defensive barrier by reducing the production of IgA in the gut.
Figures


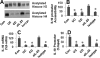

References
-
- Monteleone G., Biancone L., Marasco R., Morrone G., Marasco O., Luzza F., Pallone F. (1997) Interleukin 12 is expressed and actively released by Crohn disease intestinal lamina propria mononuclear cells. Gastroenterology 112, 1169–1178 - PubMed
-
- Fuss I. J., Neurath M., Boirivant M., Klein J. S., de la Motte C., Strong S. A., Fiocchi C., Strober W. (1996) Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn disease LP cells manifest increased secretion of IFN-γ, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J. Immunol. 157, 1261–1270 - PubMed
-
- D'Haens G. (2010) Anti-TNF treatment in Crohn disease: toward tailored therapy? Am. J. Gastroenterol. 105, 1140–1141 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

