Nanocarriers enhance Doxorubicin uptake in drug-resistant ovarian cancer cells
- PMID: 22158944
- PMCID: PMC3657469
- DOI: 10.1158/0008-5472.CAN-11-2890
Nanocarriers enhance Doxorubicin uptake in drug-resistant ovarian cancer cells
Abstract
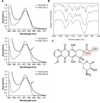
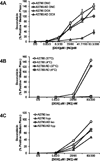
Resistance to anthracyclines and other chemotherapeutics due to P-glycoprotein (pgp)-mediated export is a frequent problem in cancer treatment. Here, we report that iron oxide-titanium dioxide core-shell nanocomposites can serve as efficient carriers for doxorubicin to overcome this common mechanism of drug resistance in cancer cells. Doxorubicin nanocarriers (DNC) increased effective drug uptake in drug-resistant ovarian cells. Mechanistically, doxorubicin bound to the TiO(2) surface by a labile bond that was severed upon acidification within cell endosomes. Upon its release, doxorubicin traversed the intracellular milieu and entered the cell nucleus by a route that evaded pgp-mediated drug export. Confocal and X-ray fluorescence microscopy and flow cytometry were used to show the ability of DNCs to modulate transferrin uptake and distribution in cells. Increased transferrin uptake occurred through clathrin-mediated endocytosis, indicating that nanocomposites and DNCs may both interfere with removal of transferrin from cells. Together, our findings show that DNCs not only provide an alternative route of delivery of doxorubicin to pgp-overexpressing cancer cells but also may boost the uptake of transferrin-tagged therapeutic agents.
©2011 AACR.
Figures




References
-
- Schondorf T, Kurbacher CM, Gohring UJ, Benz C, Becker M, Sartorius J, et al. Induction of MDR1-gene expression by antineoplastic agents in ovarian cancer cell lines. Anticancer Res. 2002 Jul-Aug;22(4):2199–2203. - PubMed
-
- Linn SC, Giaccone G. Mdr1/P-Glycoprotein Expression in Colorectal-Cancer. European Journal of Cancer. 1995 Jul-Aug;31A(7–8):1291–1294. - PubMed
-
- Yu ST, Chen TM, Tseng SY, Chen YH. Tryptanthrin inhibits MDR1 and reverses doxorubicin resistance in breast cancer cells. Biochem Bioph Res Co. 2007 Jun 22;358(1):79–84. - PubMed
-
- Shen DW, Fojo A, Chin JE, Roninson IB, Richert N, Pastan I, et al. Human multidrug-resistant cell lines: increased mdr1 expression can precede gene amplification. Science. 1986 May 2;232(4750):643–645. - PubMed
-
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacological Reviews. 2004 Jun;56(2):185–229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 CA89018/CA/NCI NIH HHS/United States
- EB002100/EB/NIBIB NIH HHS/United States
- T32 CA009560/CA/NCI NIH HHS/United States
- TL1 RR025739/RR/NCRR NIH HHS/United States
- R01 CA107467/CA/NCI NIH HHS/United States
- OD 5TL1RR025739/OD/NIH HHS/United States
- CA107467/CA/NCI NIH HHS/United States
- P30 CA060553/CA/NCI NIH HHS/United States
- U54 CA119341/CA/NCI NIH HHS/United States
- P50 CA089018/CA/NCI NIH HHS/United States
- R01 EB002100/EB/NIBIB NIH HHS/United States
- 5 T32 CA 9560-23/CA/NCI NIH HHS/United States
- U54CA119341/CA/NCI NIH HHS/United States
- CA060553/CA/NCI NIH HHS/United States
- U54 CA151880/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

