Iron and iron regulatory proteins in amoeboid microglial cells are linked to oligodendrocyte death in hypoxic neonatal rat periventricular white matter through production of proinflammatory cytokines and reactive oxygen/nitrogen species
- PMID: 22159112
- PMCID: PMC6634148
- DOI: 10.1523/JNEUROSCI.2250-11.2011
Iron and iron regulatory proteins in amoeboid microglial cells are linked to oligodendrocyte death in hypoxic neonatal rat periventricular white matter through production of proinflammatory cytokines and reactive oxygen/nitrogen species
Abstract
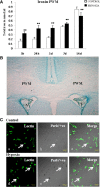
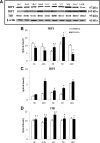
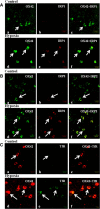
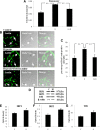
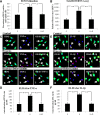
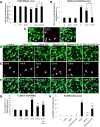
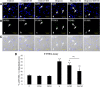
This study was aimed to examine the role of iron in causing periventricular white matter (PWM) damage following a hypoxic injury in the developing brain. Along with iron, the expression of iron regulatory proteins (IRPs) and transferrin receptor (TfR), which are involved in iron acquisition, was also examined in the PWM by subjecting 1-d-old Wistar rats to hypoxia. Apart from an increase in iron levels in PWM, Perls' iron staining showed an increase of intracellular iron in the preponderant amoeboid microglial cells (AMCs) in the tissue. In response to hypoxia, the protein levels of IRP1, IRP2, and TfR in PWM and AMCs were significantly increased. In primary microglial cultures, administration of iron chelator deferoxamine reduced the generation of iron-induced reactive oxygen and nitrogen species and proinflammatory cytokines such as tumor necrosis factor-α and interleukin-1β. Primary oligodendrocytes treated with conditioned medium from hypoxic microglia exhibited reduced glutathione levels, increased lipid peroxidation, upregulated caspase-3 expression, and reduced proliferation. This was reversed to control levels on treatment with conditioned medium from deferoxamine treated hypoxic microglia; also, there was reduction in apoptosis of oligodendrocytes. The present results suggest that excess iron derived primarily from AMCs might be a mediator of oligodendrocyte cell death in PWM following hypoxia in the neonatal brain.
Figures

Similar articles
-
Roles of activated microglia in hypoxia induced neuroinflammation in the developing brain and the retina.J Neuroimmune Pharmacol. 2013 Mar;8(1):66-78. doi: 10.1007/s11481-012-9347-2. Epub 2012 Feb 26. J Neuroimmune Pharmacol. 2013. PMID: 22367679 Review.
-
Hypoxia inducible factor-1α mediates iron uptake which induces inflammatory response in amoeboid microglial cells in developing periventricular white matter through MAP kinase pathway.Neuropharmacology. 2014 Feb;77:428-40. doi: 10.1016/j.neuropharm.2013.10.024. Epub 2013 Nov 1. Neuropharmacology. 2014. PMID: 24184387
-
Amoeboid microglia in the periventricular white matter induce oligodendrocyte damage through expression of proinflammatory cytokines via MAP kinase signaling pathway in hypoxic neonatal rats.Brain Pathol. 2008 Jul;18(3):387-400. doi: 10.1111/j.1750-3639.2008.00138.x. Epub 2008 Mar 26. Brain Pathol. 2008. PMID: 18371179 Free PMC article.
-
Expression of N-methyl D-aspartate receptor subunits in amoeboid microglia mediates production of nitric oxide via NF-κB signaling pathway and oligodendrocyte cell death in hypoxic postnatal rats.Glia. 2011 Apr;59(4):521-39. doi: 10.1002/glia.21121. Epub 2011 Jan 6. Glia. 2011. PMID: 21319220
-
Periventricular white matter damage in the hypoxic neonatal brain: role of microglial cells.Prog Neurobiol. 2009 Apr;87(4):264-80. doi: 10.1016/j.pneurobio.2009.01.003. Epub 2009 Jan 24. Prog Neurobiol. 2009. PMID: 19428957 Review.
Cited by
-
Roles of activated microglia in hypoxia induced neuroinflammation in the developing brain and the retina.J Neuroimmune Pharmacol. 2013 Mar;8(1):66-78. doi: 10.1007/s11481-012-9347-2. Epub 2012 Feb 26. J Neuroimmune Pharmacol. 2013. PMID: 22367679 Review.
-
[Research advances in the role of ferroptosis in neonatal hypoxic-ischemic brain damage].Zhongguo Dang Dai Er Ke Za Zhi. 2021 May;23(5):536-541. doi: 10.7499/j.issn.1008-8830.2102045. Zhongguo Dang Dai Er Ke Za Zhi. 2021. PMID: 34020747 Free PMC article. Review. Chinese.
-
Quercetin prevents necroptosis of oligodendrocytes by inhibiting macrophages/microglia polarization to M1 phenotype after spinal cord injury in rats.J Neuroinflammation. 2019 Nov 7;16(1):206. doi: 10.1186/s12974-019-1613-2. J Neuroinflammation. 2019. PMID: 31699098 Free PMC article.
-
Longitudinal study of multiple sclerosis lesions using ultra-high field (7T) multiparametric MR imaging.PLoS One. 2018 Sep 13;13(9):e0202918. doi: 10.1371/journal.pone.0202918. eCollection 2018. PLoS One. 2018. PMID: 30212476 Free PMC article.
-
Bone biology in postnatal Wistar rats following hypoxia-reoxygenation.Histol Histopathol. 2020 Jan;35(1):111-124. doi: 10.14670/HH-18-143. Epub 2019 Jul 3. Histol Histopathol. 2020. PMID: 31268167
References
-
- Adcock LM, Yamashita Y, Goddard-Finegold J, Smith CV. Cerebral hypoxia-ischemia increases microsomal iron in newborn piglets. Metab Brain Dis. 1996;11:359–367. - PubMed
-
- Armstrong RC. Isolation and characterization of immature oligodendrocyte lineage cells. Methods. 1998;16:282–292. - PubMed
-
- Armstrong RC, Aja T, Xiang J, Gaur S, Krebs JF, Hoang K, Bai X, Korsmeyer SJ, Karanewsky DS, Fritz LC, Tomaselli KJ. Fas-induced activation of the cell death-related protease CPP32 Is inhibited by Bcl-2 and by ICE family protease inhibitors. J Biol Chem. 1996;271:16850–16855. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
