Mucin 16 is a functional selectin ligand on pancreatic cancer cells
- PMID: 22159147
- PMCID: PMC3289508
- DOI: 10.1096/fj.11-195669
Mucin 16 is a functional selectin ligand on pancreatic cancer cells
Abstract
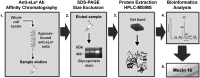
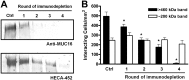
Selectins promote metastasis by mediating specific interactions between selectin ligands on tumor cells and selectin-expressing host cells in the microvasculature. Using affinity chromatography in conjunction with tandem mass spectrometry and bioinformatics tools, we identified mucin 16 (MUC16) as a novel selectin ligand expressed by metastatic pancreatic cancer cells. While up-regulated in many pancreatic cancers, the biological function of sialofucosylated MUC16 has yet to be fully elucidated. To address this, we employed blot rolling and cell-free flow-based adhesion assays using MUC16 immunopurified from pancreatic cancer cells and found that it efficiently binds E- and L- but not P-selectin. The selectin-binding determinants are sialofucosylated structures displayed on O- and N-linked glycans. Silencing MUC16 expression by RNAi markedly reduces pancreatic cancer cell binding to E- and L-selectin under flow. These findings provide a novel integrated perspective on the enhanced metastatic potential associated with MUC16 overexpression and the role of selectins in metastasis.
Figures




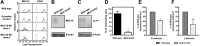
References
-
- Burdick M. M., Konstantopoulos K. (2004) Platelet-induced enhancement of LS174T colon carcinoma and THP-1 monocytoid cell adhesion to vascular endothelium under flow. Am. J. Physiol. Cell Physiol. 287, C539–547 - PubMed
-
- McCarty O. J., Mousa S. A., Bray P. F., Konstantopoulos K. (2000) Immobilized platelets support human colon carcinoma cell tethering, rolling, and firm adhesion under dynamic flow conditions. Blood 96, 1789–1797 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

