Water-soluble, deep-red fluorescent squaraine rotaxanes
- PMID: 22159917
- PMCID: PMC4015105
- DOI: 10.1039/c2ob06783h
Water-soluble, deep-red fluorescent squaraine rotaxanes
Abstract
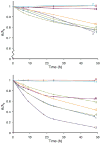
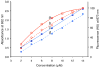
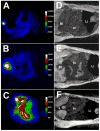
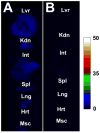
Eight fluorescent squaraine rotaxanes with deep-red absorption/emission wavelengths were prepared and assessed for chemical stability and suitability as water-soluble, fluorescent tracers. The most stable squaraine rotaxanes have four large stopper groups attached to the ends of the encapsulated squaraine, and two members of this structural class have promise as highly fluorescent tracers with rapid renal clearance and very low tissue uptake in living mice.
Figures


References
-
- Law K-Y. Chem Rev. 1993;93:449–486.
- Law K-Y. In: Organic Photochemistry. Ramamurthy V, Schanze KS, editors. Marcel Dekker; New York: 1997. p. 519.
- Das S, Thomas KG, George MV. In: Organic Photochemistry. Ramamurthy, Schanze KS, editors. Marcel Dekker; New York: 1997. p. 467.
-
- Arunkumar E, Forbes CC, Noll BC, Smith BD. J Am Chem Soc. 2005;127:3288–3289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

