Intra-amniotic LPS and antenatal betamethasone: inflammation and maturation in preterm lamb lungs
- PMID: 22160306
- PMCID: PMC3289264
- DOI: 10.1152/ajplung.00338.2011
Intra-amniotic LPS and antenatal betamethasone: inflammation and maturation in preterm lamb lungs
Abstract
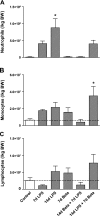
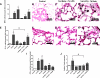
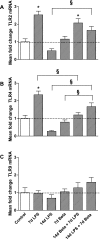
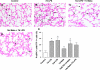
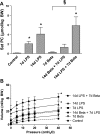
The proinflammatory stimulus of chorioamnionitis is commonly associated with preterm delivery. Women at risk of preterm delivery receive antenatal glucocorticoids to functionally mature the fetal lung. However, the effects of the combined exposures of chorioamnionitis and antenatal glucocorticoids on the fetus are poorly understood. Time-mated ewes with singleton fetuses received an intra-amniotic injection of lipopolysaccharide (LPS) either preceding or following maternal intramuscular betamethasone 7 or 14 days before delivery, and the fetuses were delivered at 120 days gestational age (GA) (term = 150 days GA). Gestation matched controls received intra-amniotic and maternal intramuscular saline. Compared with saline controls, intra-amniotic LPS increased inflammatory cells in the bronchoalveolar lavage and myeloperoxidase, Toll-like receptor 2 and 4 mRNA, PU.1, CD3, and Foxp3-positive cells in the fetal lung. LPS-induced lung maturation measured as increased airway surfactant and improved lung gas volumes. Intra-amniotic LPS-induced inflammation persisted until 14 days after exposure. Betamethasone treatment alone induced modest lung maturation but, when administered before intra-amniotic LPS, suppressed lung inflammation. Interestingly, betamethasone treatment after LPS did not counteract inflammation but enhanced lung maturation. We conclude that the order of exposures of intra-amniotic LPS or maternal betamethasone had large effects on fetal lung inflammation and maturation.
Figures

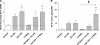
References
-
- Andrews WW, Goldenberg RL, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am J Obstet Gynecol 195: 803–808, 2006 - PubMed
-
- Bachurski CJ, Ross GF, Ikegami M, Kramer BW, Jobe AH. Intra-amniotic endotoxin increases pulmonary surfactant proteins and induces SP-B processing in fetal sheep. Am J Physiol Lung Cell Mol Physiol 280: L279–L285, 2001 - PubMed
-
- Ballard PL, Ertsey R, Gonzales LW, Gonzales J. Transcriptional regulation of human pulmonary surfactant proteins SP-B and SP-C by glucocorticoids. Am J Respir Cell Mol Biol 14: 599–607, 1996 - PubMed
-
- Banks BA, Cnaan A, Morgan MA, Parer JT, Merrill JD, Ballard PL, Ballard RA. Multiple courses of antenatal corticosteroids and outcome of premature neonates. North American Thyrotropin-Releasing Hormone Study Group. Am J Obstet Gynecol 181: 709–717, 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

