Imaging protein synthesis in cells and tissues with an alkyne analog of puromycin
- PMID: 22160674
- PMCID: PMC3258597
- DOI: 10.1073/pnas.1111561108
Imaging protein synthesis in cells and tissues with an alkyne analog of puromycin
Abstract
Synthesis of many proteins is tightly controlled at the level of translation, and plays an essential role in fundamental processes such as cell growth and proliferation, signaling, differentiation, or death. Methods that allow imaging and identification of nascent proteins are critical for dissecting regulation of translation, both spatially and temporally, particularly in whole organisms. We introduce a simple and robust chemical method to image and affinity-purify nascent proteins in cells and in animals, based on an alkyne analog of puromycin, O-propargyl-puromycin (OP-puro). OP-puro forms covalent conjugates with nascent polypeptide chains, which are rapidly turned over by the proteasome and can be visualized or captured by copper(I)-catalyzed azide-alkyne cycloaddition. Unlike methionine analogs, OP-puro does not require methionine-free conditions and, uniquely, can be used to label and assay nascent proteins in whole organisms. This strategy should have broad applicability for imaging protein synthesis and for identifying proteins synthesized under various physiological and pathological conditions in vivo.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

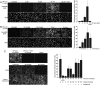
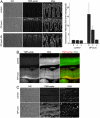
Comment in
-
Imaging of protein synthesis with puromycin.Proc Natl Acad Sci U S A. 2012 Apr 24;109(17):E989; author reply E990. doi: 10.1073/pnas.1202000109. Epub 2012 Mar 23. Proc Natl Acad Sci U S A. 2012. PMID: 22447778 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

