Antagonistic gene transcripts regulate adaptation to new growth environments
- PMID: 22160690
- PMCID: PMC3248483
- DOI: 10.1073/pnas.1111408109
Antagonistic gene transcripts regulate adaptation to new growth environments
Abstract
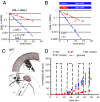
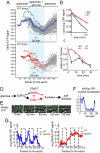
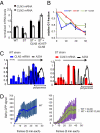
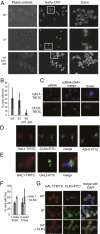
Cells have evolved complex regulatory networks that reorganize gene expression patterns in response to changing environmental conditions. These changes often involve redundant mechanisms that affect various levels of gene expression. Here, we examine the consequences of enhanced mRNA degradation in the galactose utilization network of Saccharomyces cerevisiae. We observe that glucose-induced degradation of GAL1 transcripts provides a transient growth advantage to cells upon addition of glucose. We show that the advantage arises from relief of translational competition between GAL1 transcripts and those of cyclin CLN3, a translationally regulated initiator of cell division. This competition creates a translational bottleneck that balances the production of Gal1p and Cln3p and represents a posttranscriptional control mechanism that enhances the cell's ability to adapt to changes in carbon source. We present evidence that the spatial regulation of GAL1 and CLN3 transcripts is what allows growth to be maintained during fluctuations of glucose availability. Our results provide unique insights into how cells optimize energy use during growth in a dynamic environment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

