High-throughput VDJ sequencing for quantification of minimal residual disease in chronic lymphocytic leukemia and immune reconstitution assessment
- PMID: 22160699
- PMCID: PMC3248502
- DOI: 10.1073/pnas.1118357109
High-throughput VDJ sequencing for quantification of minimal residual disease in chronic lymphocytic leukemia and immune reconstitution assessment
Abstract
The primary cause of poor outcome following allogeneic hematopoietic cell transplantation (HCT) for chronic lymphocytic leukemia (CLL) is disease recurrence. Detection of increasing minimal residual disease (MRD) following HCT may permit early intervention to prevent clinical relapse; however, MRD quantification remains an uncommon diagnostic test because of logistical and financial barriers to widespread use. Here we describe a method for quantifying CLL MRD using widely available consensus primers for amplification of all Ig heavy chain (IGH) genes in a mixture of peripheral blood mononuclear cells, followed by high-throughput sequencing (HTS) for disease-specific IGH sequence quantification. To achieve accurate MRD quantification, we developed a systematic bioinformatic methodology to aggregate cancer clone sequence variants arising from systematic and random artifacts occurring during IGH-HTS. We then compared the sensitivity of IGH-HTS, flow cytometry, and allele-specific oligonucleotide PCR for MRD quantification in 28 samples collected from 6 CLL patients following allogeneic HCT. Using amplimer libraries generated with consensus primers from patient blood samples, we demonstrate the sensitivity of IGH-HTS with 454 pyrosequencing to be 10(-5), with a high correlation between quantification by allele-specific oligonucleotide PCR and IGH-HTS (r = 0.85). From the same dataset used to quantify MRD, IGH-HTS also allowed us to profile IGH repertoire reconstitution after HCT-information not provided by the other MRD methods. IGH-HTS using consensus primers will broaden the availability of MRD quantification in CLL and other B cell malignancies, and this approach has potential for quantitative evaluation of immune diversification following transplant and nontransplant therapies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

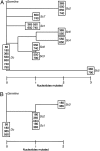

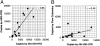

References
-
- National Cancer Institute SEER Cancer Statistics Review. 2011. Available at http://seer.cancer.gov/csr. Accessed May 13, 2011.
-
- Hallek M. State-of-the-art treatment of chronic lymphocytic leukemia. Hematology (Am Soc Hematol Educ Program) 2009;2009:440–449. - PubMed
-
- Hallek M, et al. International Workshop on Chronic Lymphocytic Leukemia Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. - PMC - PubMed
-
- Khouri IF, et al. Nonablative allogeneic stem cell transplantation for chronic lymphocytic leukemia: Impact of rituximab on immunomodulation and survival. Exp Hematol. 2004;32:28–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

