Structural basis for gating charge movement in the voltage sensor of a sodium channel
- PMID: 22160714
- PMCID: PMC3258622
- DOI: 10.1073/pnas.1118434109
Structural basis for gating charge movement in the voltage sensor of a sodium channel
Abstract
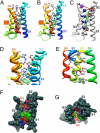
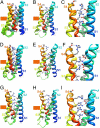
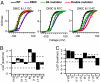
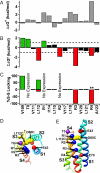
Voltage-dependent gating of ion channels is essential for electrical signaling in excitable cells, but the structural basis for voltage sensor function is unknown. We constructed high-resolution structural models of resting, intermediate, and activated states of the voltage-sensing domain of the bacterial sodium channel NaChBac using the Rosetta modeling method, crystal structures of related channels, and experimental data showing state-dependent interactions between the gating charge-carrying arginines in the S4 segment and negatively charged residues in neighboring transmembrane segments. The resulting structural models illustrate a network of ionic and hydrogen-bonding interactions that are made sequentially by the gating charges as they move out under the influence of the electric field. The S4 segment slides 6-8 Å outward through a narrow groove formed by the S1, S2, and S3 segments, rotates ∼30°, and tilts sideways at a pivot point formed by a highly conserved hydrophobic region near the middle of the voltage sensor. The S4 segment has a 3(10)-helical conformation in the narrow inner gating pore, which allows linear movement of the gating charges across the inner one-half of the membrane. Conformational changes of the intracellular one-half of S4 during activation are rigidly coupled to lateral movement of the S4-S5 linker, which could induce movement of the S5 and S6 segments and open the intracellular gate of the pore. We confirmed the validity of these structural models by comparing with a high-resolution structure of a NaChBac homolog and showing predicted molecular interactions of hydrophobic residues in the S4 segment in disulfide-locking studies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
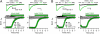



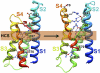
Similar articles
-
Role of arginine residues on the S4 segment of the Bacillus halodurans Na+ channel in voltage-sensing.J Membr Biol. 2004 Sep 1;201(1):9-24. doi: 10.1007/s00232-004-0701-z. J Membr Biol. 2004. PMID: 15635808
-
Models of voltage-dependent conformational changes in NaChBac channels.Biophys J. 2008 Oct;95(8):3663-76. doi: 10.1529/biophysj.108.135335. Epub 2008 Jul 18. Biophys J. 2008. PMID: 18641074 Free PMC article.
-
Tracking S4 movement by gating pore currents in the bacterial sodium channel NaChBac.J Gen Physiol. 2014 Aug;144(2):147-57. doi: 10.1085/jgp.201411210. J Gen Physiol. 2014. PMID: 25070432 Free PMC article.
-
Voltage sensors.Mol Pharmacol. 2025 Feb;107(2):100011. doi: 10.1016/j.molpha.2024.100011. Epub 2024 Dec 12. Mol Pharmacol. 2025. PMID: 40023511 Review.
-
Molecular basis of K+ channel inactivation gating.EXS. 1993;63:338-51. doi: 10.1007/978-3-0348-7265-2_18. EXS. 1993. PMID: 8380732 Review.
Cited by
-
Cannabidiol inhibits the skeletal muscle Nav1.4 by blocking its pore and by altering membrane elasticity.J Gen Physiol. 2021 May 3;153(5):e202012701. doi: 10.1085/jgp.202012701. J Gen Physiol. 2021. PMID: 33836525 Free PMC article.
-
Exploring conformational states of the bacterial voltage-gated sodium channel NavAb via molecular dynamics simulations.Proc Natl Acad Sci U S A. 2012 Dec 26;109(52):21336-41. doi: 10.1073/pnas.1218087109. Epub 2012 Nov 12. Proc Natl Acad Sci U S A. 2012. PMID: 23150565 Free PMC article.
-
Case report: A novel CACNA1S mutation associated with hypokalemic periodic paralysis.Front Neurol. 2023 Sep 29;14:1267426. doi: 10.3389/fneur.2023.1267426. eCollection 2023. Front Neurol. 2023. PMID: 37840943 Free PMC article.
-
The 70-year search for the voltage-sensing mechanism of ion channels.J Physiol. 2022 Jul;600(14):3227-3247. doi: 10.1113/JP282780. Epub 2022 Jul 6. J Physiol. 2022. PMID: 35665931 Free PMC article.
-
Structural basis for gating pore current in periodic paralysis.Nature. 2018 May;557(7706):590-594. doi: 10.1038/s41586-018-0120-4. Epub 2018 May 16. Nature. 2018. PMID: 29769724 Free PMC article.
References
-
- Hille B. Ion Channels of Excitable Membranes. 3rd Ed. Sunderland, MA: Sinauer; 2001.
-
- Catterall WA. From ionic currents to molecular mechanisms: The structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. - PubMed
-
- Yu FH, Catterall WA. The VGL-chanome: A protein superfamily specialized for electrical signaling and ionic homeostasis. Sci STKE. 2004;2004:re15. - PubMed
-
- Ren D, et al. A prokaryotic voltage-gated sodium channel. Science. 2001;294:2372–2375. - PubMed
-
- Koishi R, et al. A superfamily of voltage-gated sodium channels in bacteria. J Biol Chem. 2004;279:9532–9538. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

