Reverse signaling through the costimulatory ligand CD137L in epithelial cells is essential for natural killer cell-mediated acute tissue inflammation
- PMID: 22160719
- PMCID: PMC3252952
- DOI: 10.1073/pnas.1112256109
Reverse signaling through the costimulatory ligand CD137L in epithelial cells is essential for natural killer cell-mediated acute tissue inflammation
Abstract
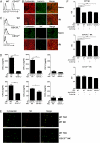
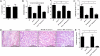
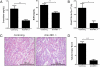
Renal ischemia-reperfusion injury (IRI) after kidney transplantation is a major cause of delayed graft function. Even though IRI is recognized as a highly coordinated and specific process, the pathways and mechanisms through which the innate response is activated are poorly understood. In this study, we used a mouse model of acute kidney IRI to examine whether the interactions of costimulatory receptor CD137 and its ligand (CD137L) are involved in the early phase of acute kidney inflammation caused by IRI. We report here that the specific expressions of CD137 on natural killer cells and of CD137L on tubular epithelial cells (TECs) are required for acute kidney IRI. Reverse signaling through CD137L in TECs results in their production of the chemokine (C-X-C motif) receptor 2 ligands CXCL1 and CXCL2 and the subsequent induction of neutrophil recruitment, resulting in a cascade of proinflammatory events during kidney IRI. Our findings identify an innate pathogenic pathway for renal IRI involving the natural killer cell-TEC-neutrophil axis, whereby CD137-CD137L interactions provide the causal contribution of epithelial cell dysregulation to renal IRI. The CD137L reverse signaling pathway in epithelial cells therefore may represent a good target for blocking the initial stage of inflammatory diseases, including renal IRI.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

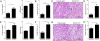

Similar articles
-
TLR2 signaling in tubular epithelial cells regulates NK cell recruitment in kidney ischemia-reperfusion injury.J Immunol. 2013 Sep 1;191(5):2657-64. doi: 10.4049/jimmunol.1300358. Epub 2013 Jul 31. J Immunol. 2013. PMID: 23904170
-
NK cells induce apoptosis in tubular epithelial cells and contribute to renal ischemia-reperfusion injury.J Immunol. 2008 Dec 1;181(11):7489-98. doi: 10.4049/jimmunol.181.11.7489. J Immunol. 2008. PMID: 19017938
-
Osteopontin expressed in tubular epithelial cells regulates NK cell-mediated kidney ischemia reperfusion injury.J Immunol. 2010 Jul 15;185(2):967-73. doi: 10.4049/jimmunol.0903245. Epub 2010 Jun 14. J Immunol. 2010. PMID: 20548025
-
Metabolic Flexibility and Innate Immunity in Renal Ischemia Reperfusion Injury: The Fine Balance Between Adaptive Repair and Tissue Degeneration.Front Immunol. 2020 Jul 7;11:1346. doi: 10.3389/fimmu.2020.01346. eCollection 2020. Front Immunol. 2020. PMID: 32733450 Free PMC article. Review.
-
Reverse signaling through the co-stimulatory ligand, CD137L, as a critical mediator of sterile inflammation.Mol Cells. 2012 Jun;33(6):533-7. doi: 10.1007/s10059-012-0077-3. Epub 2012 Apr 20. Mol Cells. 2012. PMID: 22526397 Free PMC article. Review.
Cited by
-
Kidney disease models: tools to identify mechanisms and potential therapeutic targets.Zool Res. 2018 Mar 18;39(2):72-86. doi: 10.24272/j.issn.2095-8137.2017.055. Zool Res. 2018. PMID: 29515089 Free PMC article. Review.
-
Innate immune cells in acute and chronic kidney disease.Nat Rev Nephrol. 2025 Jul;21(7):464-482. doi: 10.1038/s41581-025-00958-x. Epub 2025 Apr 22. Nat Rev Nephrol. 2025. PMID: 40263532 Review.
-
CD137 signaling aggravates myocardial ischemia-reperfusion injury by inhibiting mitophagy mediated NLRP3 inflammasome activation.J Geriatr Cardiol. 2023 Mar 28;20(3):223-237. doi: 10.26599/1671-5411.2023.03.004. J Geriatr Cardiol. 2023. PMID: 37091265 Free PMC article.
-
Protective and pathogenic functions of innate lymphoid cells in transplantation.Clin Exp Immunol. 2023 Jul 5;213(1):23-39. doi: 10.1093/cei/uxad050. Clin Exp Immunol. 2023. PMID: 37119279 Free PMC article. Review.
-
Expression of Tumor-mediated CD137 ligand in human colon cancer indicates dual signaling effects.Oncoimmunology. 2019 Sep 6;8(12):e1651622. doi: 10.1080/2162402X.2019.1651622. eCollection 2019. Oncoimmunology. 2019. PMID: 31741755 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

