Human monocytes are severely impaired in base and DNA double-strand break repair that renders them vulnerable to oxidative stress
- PMID: 22160723
- PMCID: PMC3248544
- DOI: 10.1073/pnas.1111919109
Human monocytes are severely impaired in base and DNA double-strand break repair that renders them vulnerable to oxidative stress
Abstract
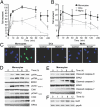
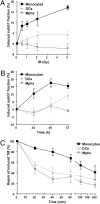
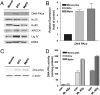
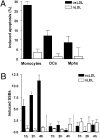
Monocytes are key players in the immune system. Crossing the blood barrier, they infiltrate tissues and differentiate into (i) macrophages that fight off pathogens and (ii) dendritic cells (DCs) that activate the immune response. A hallmark of monocyte/macrophage activation is the generation of reactive oxygen species (ROS) as a defense against invading microorganisms. How monocytes, macrophages, and DCs in particular respond to ROS is largely unknown. Here we studied the sensitivity of primary human monocytes isolated from peripheral blood and compared them with macrophages and DCs derived from them by cytokine maturation following DNA damage induced by ROS. We show that monocytes are hypersensitive to ROS, undergoing excessive apoptosis. These cells exhibited a high yield of ROS-induced DNA single- and double-strand breaks and activation of the ATR-Chk1-ATM-Chk2-p53 pathway that led to Fas and caspase-8, -3, and -7 activation, whereas macrophages and DCs derived from them were protected. Monocytes are also hypersensitive to ionizing radiation and oxidized low-density lipoprotein. The remarkable sensitivity of monocytes to oxidative stress is caused by a lack of expression of the DNA repair proteins XRCC1, ligase IIIα, poly(ADP-ribose) polymerase-1, and catalytic subunit of DNA-dependent protein kinase (DNA-PK(cs)), causing a severe DNA repair defect that impacts base excision repair and double-strand break repair by nonhomologous end-joining. During maturation of monocytes into macrophages and DCs triggered by the cytokines GM-CSF and IL-4, these proteins become up-regulated, making macrophages and DCs repair-competent and ROS-resistant. We propose that impaired DNA repair in monocytes plays a role in the regulation of the monocyte/macrophage/DC system following ROS exposure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hume DA. The mononuclear phagocyte system. Curr Opin Immunol. 2006;18:49–53. - PubMed
-
- Enk AH, Jonuleit H, Saloga J, Knop J. Dendritic cells as mediators of tumor-induced tolerance in metastatic melanoma. Int J Cancer. 1997;73:309–316. - PubMed
-
- Durackova Z. Some current insights into oxidative stress. Physiol Res. 2010;59:459–469. - PubMed
-
- Fialkow L, Wang Y, Downey GP. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic Biol Med. 2007;42:153–164. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

