Sound-evoked olivocochlear activation in unanesthetized mice
- PMID: 22160753
- PMCID: PMC3298614
- DOI: 10.1007/s10162-011-0306-z
Sound-evoked olivocochlear activation in unanesthetized mice
Abstract
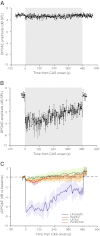
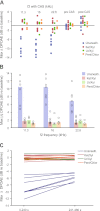
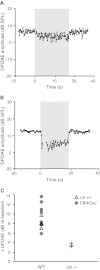
Genetic tools available for the mouse make it a powerful model to study the modulation of cochlear function by descending control systems. Suppression of distortion product otoacoustic emission (DPOAE) amplitude by contralateral acoustic stimulation (CAS) provides a robust tool for noninvasively monitoring the strength of descending modulation, yet investigations in mice have been performed infrequently and only under anesthesia, a condition likely to reduce olivocochlear activation. Here, we characterize the contralateral olivocochlear reflex in the alert, unanesthetized mouse. Head-fixed mice were restrained between two closed acoustic systems, while an artifact rejection protocol minimized contamination from self-generated sounds and movements. In mice anesthetized with pentobarbital, ketamine or urethane, CAS at 80 dB SPL evoked, on average, a <1-dB change in DPOAE amplitude. In contrast, the mean CAS-induced DPOAE suppression in unanesthetized mice was nearly 8 dB. Experiments in mice with targeted deletion of the α9 subunit of the nicotinic acetylcholine receptor confirmed the contribution of the medial olivocochlear efferents to this phenomenon. These findings demonstrate the utility of the CAS assay in the unanesthetized mouse and highlight the adverse effects of anesthesia when probing the functional status of descending control pathways within the auditory system.
Figures

References
-
- Bauerle P, Von der Behrens W, Kossl M, Gaese BH. Stimulus-specific adaptation in the gerbil primary auditory thalamus is the result of a fast frequency-specific habituation and is regulated by the corticofugal system. J Neurosci. 2011;31:9708–9722. doi: 10.1523/JNEUROSCI.5814-10.2011. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

