Prediction of drug response and safety in clinical practice
- PMID: 22160757
- PMCID: PMC3550218
- DOI: 10.1007/s13181-011-0198-7
Prediction of drug response and safety in clinical practice
Abstract
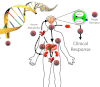
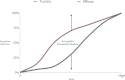
Many clinicians hoped that the completion of the Human Genome Project would result in "individualized drug therapy," i.e., determining the right medication at the right dose 100% of the time based upon the individual's genetics. The pharmacogenomic prediction of drug efficacy and safety has not become a reality due to continuing realization of the complexity dictating the human-drug interaction. New methods of metabolomics, proteomics, and transcriptomics that account for this complexity hold promise for translational researchers hoping to increase drug efficacy and decrease drug toxicity.
Figures
References
-
- Ieiri I, Higuchi S, Sugiyama Y. Genetic polymorphisms of uptake (OATP1B1, 1B3) and efflux (MRP2, BCRP) transporters: implications for inter-individual differences in the pharmacokinetics and pharmacodynamics of statins and other clinically relevant drugs. Expert Opin Drug Metab Toxicol. 2009;5(7):703–729. doi: 10.1517/17425250902976854. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

