Persistent disruption of mitochondrial homeostasis after acute kidney injury
- PMID: 22160772
- PMCID: PMC3340936
- DOI: 10.1152/ajprenal.00035.2011
Persistent disruption of mitochondrial homeostasis after acute kidney injury
Abstract
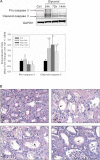
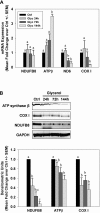
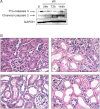
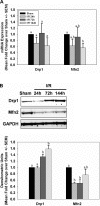
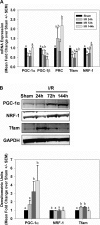
While mitochondrial dysfunction is a pathological process that occurs after acute kidney injury (AKI), the state of mitochondrial homeostasis during the injury and recovery phases of AKI remains unclear. We examined markers of mitochondrial homeostasis in two nonlethal rodent AKI models. Myoglobinuric AKI was induced by glycerol injection into rats, and mice were subjected to ischemic AKI. Animals in both models had elevated serum creatinine, indicative of renal dysfunction, 24 h after injury which partially recovered over 144 h postinjury. Markers of proximal tubule function/injury, including neutrophil gelatinase-associated lipocalin and urine glucose, did not recover during this same period. The persistent pathological state was confirmed by sustained caspase 3 cleavage and evidence of tubule dilation and brush-border damage. Respiratory proteins NDUFB8, ATP synthase β, cytochrome c oxidase subunit I (COX I), and COX IV were decreased in both injury models and did not recover by 144 h. Immunohistochemical analysis confirmed that COX IV protein was progressively lost in proximal tubules of the kidney cortex after ischemia-reperfusion (I/R). Expression of mitochondrial fission protein Drp1 was elevated after injury in both models, whereas the fusion protein Mfn2 was elevated after glycerol injury but decreased after I/R AKI. LC3-I/II expression revealed that autophagy increased in both injury models at the later time points. Markers of mitochondrial biogenesis, such as PGC-1α and PRC, were elevated in both models. These findings reveal that there is persistent disruption of mitochondrial homeostasis and sustained tubular damage after AKI, even in the presence of mitochondrial recovery signals and improved glomerular filtration.
Figures







References
-
- Bonventre JV. Mechanisms of ischemic acute renal failure. Kidney Int 43: 1160–1178, 1993 - PubMed
-
- Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 14: 2199–2210, 2003 - PubMed
-
- Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med 361: 62–72, 2009 - PubMed
-
- Cameron JS. Allergic interstitial nephritis: clinical features and pathogenesis. Q J Med 66: 97–115, 1988 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

