Inhaled corticosteroids versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease
- PMID: 22161409
- PMCID: PMC6494276
- DOI: 10.1002/14651858.CD007033.pub3
Inhaled corticosteroids versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease
Abstract
Background: Long-acting beta(2)-agonists and inhaled corticosteroids can be used as maintenance therapy by patients with moderate to severe chronic obstructive pulmonary disease. These interventions are often taken together in a combination inhaler. However, the relative added value of the two individual components is unclear.
Objectives: To determine the relative effects of inhaled corticosteroids (ICS) compared to long-acting beta(2)-agonists (LABA) on clinical outcomes in patients with stable chronic obstructive pulmonary disease.
Search methods: We searched the Cochrane Airways Group Specialised Register of trials (latest search August 2011) and reference lists of articles.
Selection criteria: We included randomised controlled trials comparing inhaled corticosteroids and long-acting beta(2)-agonists in the treatment of patients with stable chronic obstructive pulmonary disease.
Data collection and analysis: Three authors independently assessed trials for inclusion and then extracted data on trial quality, study outcomes and adverse events. We also contacted study authors for additional information.
Main results: We identified seven randomised trials (5997 participants) of good quality with a duration of six months to three years. All of the trials compared ICS/LABA combination inhalers with LABA and ICS as individual components. Four of these trials included fluticasone and salmeterol monocomponents and the remaining three included budesonide and formoterol monocomponents. There was no statistically significant difference in our primary outcome, the number of patients experiencing exacerbations (odds ratio (OR) 1.22; 95% CI 0.89 to 1.67), or the rate of exacerbations per patient year (rate ratio (RR) 0.96; 95% CI 0.89 to 1.02) between inhaled corticosteroids and long-acting beta(2)-agonists. The incidence of pneumonia, our co-primary outcome, was significantly higher among patients on inhaled corticosteroids than on long-acting beta(2)-agonists whether classified as an adverse event (OR 1.38; 95% CI 1.10 to 1.73) or serious adverse event (Peto OR 1.48; 95% CI 1.13 to 1.93). Results of the secondary outcomes analysis were as follows. Mortality was higher in patients on inhaled corticosteroids compared to patients on long-acting beta(2)-agonists (Peto OR 1.17; 95% CI 0.97 to 1.42), although the difference was not statistically significant. Patients treated with beta(2)-agonists showed greater improvements in pre-bronchodilator FEV(1) compared to those treated with inhaled corticosteroids (mean difference (MD) 18.99 mL; 95% CI 0.52 to 37.46), whilst greater improvements in health-related quality of life were observed in patients receiving inhaled corticosteroids compared to those receiving long-acting beta(2)-agonists (St George's Respiratory Questionnaire (SGRQ) MD -0.74; 95% CI -1.42 to -0.06). In both cases the differences were statistically significant but rather small in magnitude. There were no statistically significant differences between ICS and LABA in the number of hospitalisations due to exacerbations, number of mild exacerbations, peak expiratory flow, dyspnoea, symptoms scores, use of rescue medication, adverse events, all cause hospitalisations, or withdrawals from studies.
Authors' conclusions: Placebo-controlled trials have established the benefits of both long-acting beta-agonist and inhaled corticosteroid therapy for COPD patients as individual therapies. This review, which included trials allowing comparisons between LABA and ICS, has shown that the two therapies confer similar benefits across the majority of outcomes, including the frequency of exacerbations and mortality. Use of long-acting beta-agonists appears to confer a small additional benefit in terms of improvements in lung function compared to inhaled corticosteroids. On the other hand, inhaled corticosteroid therapy shows a small advantage over long-acting beta-agonist therapy in terms of health-related quality of life, but inhaled corticosteroids also increase the risk of pneumonia. This review supports current guidelines advocating long-acting beta-agonists as frontline therapy for COPD, with regular inhaled corticosteroid therapy as an adjunct in patients experiencing frequent exacerbations.
Conflict of interest statement
Sally Spencer has had consultancy arrangements with GlaxoSmithKline who make long‐acting beta2‐agonists and inhaled corticosteroids. She has also previously been funded by GlaxoSmithKline. David Evans, Christopher Cates and Charlotta Karner do not have any known conflicts of interest.
Figures
















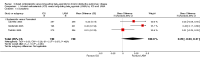





Update of
-
Inhaled corticosteroids versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2011 Oct 5;(10):CD007033. doi: 10.1002/14651858.CD007033.pub2. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2011 Dec 07;(12):CD007033. doi: 10.1002/14651858.CD007033.pub3. PMID: 21975759 Updated.
References
References to studies included in this review
Calverley 2003 {published and unpublished data}
-
- Borgstrom L, Asking L, Olsson H, Peterson S. Lack of interaction between disease severity and therapeutic response with budesonide/formoterol in a single inhaler [Abstract]. American Thoracic Society 100th International Conference, May 21‐26. 2004:C22 [Poster 505].
-
- Calverley PM, Bonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. European Respiratory Journal 2003;22(6):912‐9. - PubMed
-
- Calverley PMA. Effect of budesonide/formoterol on severe exacerbations and lung function in moderate to severe COPD. Thorax 2002;57 Suppl III:iii44.
-
- Calverley PMA, Cseke Z, Peterson S. Budesonide/formoterol reduces the use of oral corticosteroids in the treatment of COPD [Abstract]. European Respiratory Journal 2003;22 Suppl 45:P436. - PubMed
-
- Calverley PMA, Kuna P, Olsson H. COPD exacerbations are reduced by budesonide/formoterol in a single inhaler [Abstract]. European Respiratory Journal 2003;22 Suppl 45:P1587.
Hanania 2003 {published and unpublished data}
-
- Hanania NA, Darken P, Horstman D, Reisner C, Lee B, Davis S, et al. The efficacy and safety of fluticasone propionate (250 micro g)/salmeterol (50 micro g) combined in the diskus inhaler for the treatment of COPD. Chest 2003;124(3):834‐43. - PubMed
-
- Hanania NA, Knobil K, Watkins M, Wire P, Yates J, Darken P. Salmeterol and fluticasone propionate therapy administered by a single diskus in patients with COPD. Chest. 2002:S129.
-
- Hanania NA, Ramsdell J, Payne K, Davis S, Horstman D, Lee B, et al. Improvements in airflow and dyspnea in COPD patients following 24 weeks treatment with salmeterol 50mcg and fluticasone propionate 250mcg alone or in combination via the diskus. American Journal of Respiratory and Critical Care Medicine 2001;163 Suppl(5):A279.
-
- Horstman D, Darken P, Davis S, Lee B. Improvements in FEV1 and symptoms in poorly reversible COPD patients following treatment with salmeterol 50mcg/fluticasone propionate 250mcg combination [Abstract]. European Respiratory Journal 2003;22 Suppl 45:P434.
-
- Mahler DA, Darken P, Brown CP, Knobil K. Predicting lung function responses to combination therapy in chronic obstructive pulmonary disease (COPD) [Abstract]. National COPD Conference; Arlington, Virginia. 2003:Abstract 1081.
Mahler 2002 {published and unpublished data}
-
- Mahler DA, Darken P, Brown CP, Knobil K. Predicting lung function responses to combination therapy in chronic obstructive pulmonary disease (COPD). http://www.abstracts2view.com 2003.
-
- Mahler DA, Wire P, Horstman D, Chang CN, Yates J, Fischer T, et al. Effectiveness of fluticasone propionate and salmeterol combination delivered via the diskus device in the treatment of chronic obstructive pulmonary disease. American Journal of Respiratory Critical Care Medicine 2002;166(8):1084‐91. - PubMed
-
- SFCA3006. A randomized, double‐blind, placebo‐controlled, parallel‐group trial evaluating the safety and efficacy of the DISKUS formulations of Salmeterol (SAL) 50mcg BID and Fluticasone Propionate (FP) 500mcg BID individually and in combination as Salmeterol 50mcg/Fluticasone Propionate 500mcg BID (SFC 50/500) compared to placebo in COPD subjects. GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
-
- Spencer M, Wire P, Lee B, Chang CN, Darken P, Horstman D. Patients with COPD using salmeterol/fluticasone propionate combination therapy experience improved quality of life. European Respiratory Journal 2003;22 Suppl 45:51.
-
- Spencer MD, Anderson JA. Salmeterol/fluticasone combination produces clinically important benefits in dyspnea and fatigue [Abstract]. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California; 2005. 2005:B93 [Poster 308].
Szafranski 2003 {published and unpublished data}
-
- Anderson P. Budesonide/formoterol in a single inhaler (Symbicort) provides early and sustained improvement in lung function in moderate to severe COPD [Abstract]. Thorax 2002;57 Suppl iii:43.
-
- Borgstrom L, Asking L, Olsson H, Peterson S. Lack of interaction between disease severity and therapeutic response with budesonide/formoterol in a single inhaler [Abstract]. American Thoracic Society 100th International Conference, May 21‐26. 2004:C22 [Poster 505].
-
- Calverley P, Pauwels R, Lofdahl CG, Svensson K, Higenbottam T, et al. Relationship between respiratory symptoms and medical treatment in exacerbations of COPD. European Respiratory Journal 2005;26(3):406‐13. - PubMed
-
- Calverley PMA. Effect of budesonide/formoterol on severe exacerbations and lung function in moderate to severe COPD. Thorax. BTS Winter meeting 2002:S145.
-
- Calverley PMA, Szafranski W, Andersson A. Budesonide/formoterol is a well‐tolerated long term maintenance therapy for COPD. European Respiratory Journal 2005;26 Suppl 49:Poster 1917.
Tashkin 2008 {unpublished data only}
-
- AstraZeneca. A 6‐month double‐blind, double‐dummy, randomized, parallel group, multicenter efficacy & safety study of SYMBICORT® pMDI 2 x 160/4.5 μg & 80/4.5 μg bid compared to formoterol TBH, budesonide pMDI (& the combination) & placebo in COPD Patients (SHINE) (study number D589900002). www.astrazenecaclinicaltrials.com 2007.
-
- Bleecker ER, Meyers DA, Bailey WC, Sims AM, Bujac SR, Goldman M, et al. Effect Of ß2‐Adrenergic Receptor Gene Polymorphism Gly16Arg On Response To Budesonide/Formoterol Pressurized Metered‐Dose Inhaler In Chronic Obstructive Pulmonary Disease [Abstract]. American Journal of Respiratory and Critical Care Medicine 2011; Vol. 183, issue Meeting Abstracts:A4086.
-
- Tashkin DP, Rennard SI, Martin P, Ramachandran S, Martin UJ, Silkoff PE, et al. Efficacy and safety of budesonide and formoterol in one pressurized metered‐dose inhaler in patients with moderate to very severe chronic obstructive pulmonary disease: results of a 6‐month randomized clinical trial. Drugs. 2008/09/10 2008; Vol. 68, issue 14:1975‐2000. [0012‐6667: (Print)] - PubMed
TORCH 2007 {published and unpublished data}
-
- Briggs AH, GHA, L‐OG, SM, CPMA, JPW, et al. Is treatment with ICS and LABA cost‐effective for COPD? Multinational economic analysis of the TORCH study. European Respiratory Journal 2010; Vol. 35, issue 3:532‐9. - PubMed
-
- Calverley P, Celli B, Anderson JA, Ferguson GT, Jenkins C, Jones PW, et al. The TOwards a Revolution in COPD Health (TORCH) study: salmeterol/fluticasone propionate improves survival in COPD over three years. Respirology 2006;11 Suppl 5:A149.
-
- Calverley PM, AJA, CB, FGT, JC, JPW, et al. Cardiovascular events in patients with COPD: TORCH study results. Thorax 2010; Vol. 65, issue 8:719‐25. - PubMed
-
- Calverley PM, Celli B, Anderson JA, Ferguson GT, Jenkins C, Jones PW, et al. The towards a revolution in COPD health (TORCH) study: fluticasone propionate /salmeterol improves survival in COPD over three years. Chest 2006;130(4):122s.
-
- Calverley PMA, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. New England Journal of Medicine 2007;356(8):775‐89. - PubMed
TRISTAN 2003 {published and unpublished data}
-
- Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2003;361(9356):449‐56. - PubMed
-
- Calverley PMA, Pauwels RA, Vestbo J, Jones PW, Pride NB, Gulsvik A, et al. Clinical improvements with salmeterol / fluticasone propionate combination in differing severities of COPD. http://www.abstracts2view.com 2003:A035 [Poster D50].
-
- Calverley PMA, Pauwels RA, Vestbo J, Jones PW, Pride NB, Gulsvik A, et al. Salmeterol/fluticasone propionate combination for one year provides greater clinical benefit than its individual components. Proceedings of the 98th International American Thoracic Society Conference http://www.abstracts‐on‐line.com/abstracts/ATS. 2002:A98 [Poster 306].
-
- Calverly PMA, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Safety of salmeterol/fluticasone propionate combination in the treatment of chronic obstructive pulmonary disease. European Respiratory Journal 2002;20 Suppl 38:242 [P1572].
-
- Hunjan MK, Chandler F. Numbers needed to treat (NNT) to avoid an exacerbation in patients with chronic obstructive pulmonary disease (COPD) using salmeterol/fluticasone propionate combination (SFC) and associated costs [Abstract]. American Thoracic Society 100th International Conference, May 21‐26. 2004:D22 [Poster 503].
References to studies excluded from this review
Barnes 2005 {published data only}
-
- Barnes NC, Qui Y, Pavord I, Parker D, Johnson M, Jeffery PK. Salmeterol/fluticasone propionate (SFC) anti‐inflammatory effects in COPD. American Thoracic Society International Conference. 2005:B93 [Poster 320].
Cazzola 2003 {published data only}
-
- Cazzola M, Salzillo A, Giglio C, Noschee P, D'Amato G. Formoterol/budesonide (FBC) in the treatment of acute exacerbations of COPD. European Respiratory Journal 2003;22 Suppl 45:P1859.
-
- Cazzola M, Salzillo A, Noschese P, Giglio C, Amato GD. Efficacy of a combination therapy with single inhaler budesonide/formoterol in the treatment of acute exacerbations of COPD. European Respiratory Journal 2004;24 Suppl 48:252.
Della Cioppa 2001 {published data only}
-
- Della Cioppa G, Byrne A, Till D, Wood R. Formoterol demonstrates bronchodilator efficacy in patients with reversible or poorly reversible chronic obstructive pulmonary disease. European Respiratory Journal 2001;18 Suppl 33:177.
-
- Greefhorst APM, Thomson MH, Byrne A, Till D. The efficacy of formoterol in patients with chronic obstructive pulmonary disease (COPD) is not influenced by concomitant corticosteroid use. European Respiratory Journal 2001;18 Suppl 33:515.
-
- Kristufek P, Levine B, Della Cioppa G, Byrne A, Till D. Bronchodilatory effects of formoterol in patients with chronic obstructive pulmonary disease (COPD) are not influenced by concomitant corticosteroid use. European Respiratory Journal 2001;18 Suppl 33:514.
Gosman 2006 {published data only}
-
- Gosman MME, Lapperre TS, Snoeck‐Stroband JB, Jansen DF, Kerstjens HAM, Hiemstra PS, et al. Effect of 6 months therapy with inhaled fluticasone propionate (FP) with or without salmeterol (S) on bronchial inflammation in COPD. Proceedings of the American Thoracic Society 2006;A111:Poster J15.
Jiang 2009 {published data only}
-
- Jiang Y, XW, WH, ZH, ZY, XX. Effects of salmeterol/fluticasone propionate on inflammation and innate immunity in COPD [Abstract]. Respirology 2009; Vol. 14 Suppl 3:A159 [OS 25–03].
Lyseng‐Williamson 2002 {published data only}
-
- Lyseng‐Williamson KA, Keating GM. Inhaled salmeterol/fluticasone propionate combination in chronic obstructive pulmonary disease. American Journal of Respiratory Medicine 2002;1(4):273‐82. - PubMed
Mittmann 2010 {published data only}
-
- Mittmann N, HP, MC, BL, WT. Cost‐effectiveness of budesonide/formoterol added to tiotropium in COPD patients in Canada, Australia and Sweden [Abstract]. European Respiratory Society Annual Congress, Barcelona, Spain, September 18‐22 2010:[5183].
Nungtjik 2009 {published data only}
-
- Nungtjik AK, MH, YF, AB. Efficacy of combination of fluticasone propionate/salmeterol in a single inhaler (Diskus) on chronic obstructive pulmonary disease [Abstract]. Respirology 2009; Vol. 14 Suppl 3:A268 [PO 033].
Reynolds 2004 {published data only}
-
- Reynolds NA, Perry CM, Keating GM. Budesonide/formoterol in chronic obstructive pulmonary disease. Drugs 2004;64(4):431‐41. - PubMed
Tzani 2010 {published data only}
-
- Tzani P, CE, NG, CE, AM, CA, et al. Reduction in air trapping and dyspnea with an extrafine combination of beclomethasone and formoterol in COPD [Abstract]. European Respiratory Society Annual Congress, Barcelona, Spain, September 18‐22 2010:[P1204].
Worth 2009 {published data only}
-
- Worth H, FK, PS, NU, MH. Budesonide/formoterol improves exercise tolerance compared with placebo and formoterol in COPD patients [Abstract]. European Respiratory Society Annual Congress, Vienna, Austria, September 12‐16 2009:[201].
Additional references
Agarwal 2010
-
- Agarwal R, Aggarwal AN, Gupta D, Jindal SK. Inhaled corticosteroids vs placebo for preventing COPD exacerbations. Chest 2010;137(2):318‐25. - PubMed
Appleton 2006
ATS
-
- Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. American Thoracic Society. American Journal of Respiratory and Critical Care Medicine. 1995/11/01 1995; Vol. 152, issue 5 Pt 2:S77‐121. [1073‐449X: (Print)] - PubMed
Boyd 1997
-
- Boyd G, Morice A, Pounsford J, Sibert M, Peslis N, Crawford C. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD). European Respiratory Journal 1997;10:815‐21. - PubMed
Burge 2000
Calverley 2003 a
-
- Calverley PMA, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2003;361:449‐56. - PubMed
Calverley 2007
-
- Calverley PM, Anderson JA, Bartolome C, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. New England Journal of Medicine 2007;356(8):775‐89. - PubMed
ERS
-
- Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. European Respiratory Journal. 1993/03/01 1993; Vol. 16 Suppl:5‐40. [0904‐1850: (Print)] - PubMed
GOLD 2010
-
- Global Strategy for the Diagnosis, Management, and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD). Available from http://www.goldcopd.com 2010.
Higgins 2008
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, Available from www.cochrane‐handbook.org, 2008.
Jones 1997
-
- Jones PW, Bosh TK. Quality of life changes in COPD patients treated with salmeterol. American Journal of Respiratory and Critical Care Medicine 1997;155:1283‐9. - PubMed
Keene 2008
-
- Keene ON, CPM, JPW, VJ, AJA. Statistical analysis of exacerbation rates in COPD: TRISTAN and ISOLDE revisited. European Respiratory Journal 2008;32(1):17‐24. [1399‐3003: (Electronic)] - PubMed
Lung Health 2000
-
- The Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. New England Journal of Medicine 2000;343:1902‐9. - PubMed
Nannini 2007
Nannini 2007a
Nannini 2007b
-
- Nannini Luis J, Cates Christopher J, Lasserson Toby J, Poole P. Combined corticosteroid and long‐acting beta‐agonist in one inhaler versus long‐acting beta‐agonists for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006829] - DOI - PubMed
NICE 2010
-
- National Clinical Guideline Centre. Chronic obstructive pulmonary disease: Management of chronic obstructive pulmonary disease in adults in primary and secondary care. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. London: National Clinical Guideline Centre, 2010. [URL: http://guidance.nice.org.uk/CG101/Guidance/pdf/English]
Pauwels 1999
-
- Pauwels RA, Lofdahl CG, Laitinen LA, Schouten JP, Postma DS, Pride NB, et al. Long‐term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. New England Journal of Medicine 1999;340(25):1948‐53. - PubMed
RevMan 5 [Computer program]
-
- Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration. Review Manager (RevMan) Version 5.1. Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration, 2008.
Rodrigo 2008
-
- Rodrigo GJ, Nannini LJ, Rodríguez‐Roisin R. Safety of long‐acting β‐agonists in stable COPD*. Chest 2008;133(5):1079‐87. - PubMed
Rodrigo 2009
-
- Rodrigo GJ, Castro‐Rodriguez JA, Plaza V. Safety and Efficacy of Combined Long‐Acting β‐Agonists and Inhaled Corticosteroids vs Long‐Acting β‐Agonists Monotherapy for Stable COPD. Chest 2009;136(4):1029‐38. - PubMed
Singh 2010
Spencer 2001
-
- Spencer S, Calverley PMA, Burge PS, Jones PW. Health status deterioration in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2001;163:122‐8. - PubMed
Spencer 2003
Vestbo 1999
-
- Vestbo J, Sorensen T, Lange P, Brix A, Torre P, Viskum K. Long‐term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. The Lancet 1999;353:1819‐23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

