Intermittent iron supplementation for improving nutrition and development in children under 12 years of age
- PMID: 22161444
- PMCID: PMC4547491
- DOI: 10.1002/14651858.CD009085.pub2
Intermittent iron supplementation for improving nutrition and development in children under 12 years of age
Abstract
Background: Approximately 600 million children of preschool and school age are anaemic worldwide. It is estimated that half of the cases are due to iron deficiency. Consequences of iron deficiency anaemia during childhood include growth retardation, reduced school achievement, impaired motor and cognitive development, and increased morbidity and mortality. The provision of daily iron supplements is a widely used strategy for improving iron status in children but its effectiveness has been limited due to its side effects, which can include nausea, constipation or staining of the teeth. As a consequence, intermittent iron supplementation (one, two or three times a week on non-consecutive days) has been proposed as an effective and safer alternative to daily supplementation.
Objectives: To assess the effects of intermittent iron supplementation, alone or in combination with other vitamins and minerals, on nutritional and developmental outcomes in children from birth to 12 years of age compared with a placebo, no intervention or daily supplementation.
Search methods: We searched the following databases on 24 May 2011: CENTRAL (2011, Issue 2), MEDLINE (1948 to May week 2, 2011), EMBASE (1980 to 2011 Week 20), CINAHL (1937 to current), POPLINE (all available years) and WHO International Clinical Trials Registry Platform (ICTRP). On 29 June 2011 we searched all available years in the following databases: SCIELO, LILACS, IBECS and IMBIOMED. We also contacted relevant organisations (on 3 July 2011) to identify ongoing and unpublished studies.
Selection criteria: Randomised and quasi-randomised trials with either individual or cluster randomisation. Participants were children under the age of 12 years at the time of intervention with no specific health problems. The intervention assessed was intermittent iron supplementation compared with a placebo, no intervention or daily supplementation.
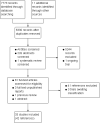
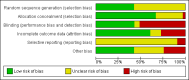
Data collection and analysis: Two authors independently assessed the eligibility of studies against the inclusion criteria, extracted data from included studies and assessed the risk of bias of the included studies.
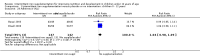
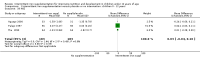
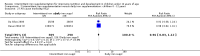
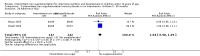
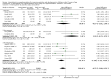
Main results: We included 33 trials, involving 13,114 children (˜49% females) from 20 countries in Latin America, Africa and Asia. The methodological quality of the trials was mixed.Nineteen trials evaluated intermittent iron supplementation versus no intervention or a placebo and 21 studies evaluated intermittent versus daily iron supplementation. Some of these trials contributed data to both comparisons. Iron alone was provided in most of the trials.Fifteen studies included children younger than 60 months; 11 trials included children 60 months and older, and seven studies included children in both age categories. One trial included exclusively females. Seven trials included only anaemic children; three studies assessed only non-anaemic children, and in the rest the baseline prevalence of anaemia ranged from 15% to 90%.In comparison with receiving no intervention or a placebo, children receiving iron supplements intermittently have a lower risk of anaemia (average risk ratio (RR) 0.51, 95% confidence interval (CI) 0.37 to 0.72, ten studies) and iron deficiency (RR 0.24, 95% CI 0.06 to 0.91, three studies) and have higher haemoglobin (mean difference (MD) 5.20 g/L, 95% CI 2.51 to 7.88, 19 studies) and ferritin concentrations (MD 14.17 µg/L, 95% CI 3.53 to 24.81, five studies).Intermittent supplementation was as effective as daily supplementation in improving haemoglobin (MD -0.60 g/L, 95% CI -1.54 to 0.35, 19 studies) and ferritin concentrations (MD -4.19 µg/L, 95% CI -9.42 to 1.05, 10 studies), but increased the risk of anaemia in comparison with daily iron supplementation (RR 1.23, 95% CI 1.04 to1.47, six studies). Data on adherence were scarce and it tended to be higher among those children receiving intermittent supplementation, although this result was not statistically significant.We did not identify any differential effect of the type of intermittent supplementation regimen (one, two or three times a week), the total weekly dose of elemental iron, the nutrient composition, whether recipients were male or female or the length of the intervention.
Authors' conclusions: Intermittent iron supplementation is efficacious to improve haemoglobin concentrations and reduce the risk of having anaemia or iron deficiency in children younger than 12 years of age when compared with a placebo or no intervention, but it is less effective than daily supplementation to prevent or control anaemia. Intermittent supplementation may be a viable public health intervention in settings where daily supplementation has failed or has not been implemented. Information on mortality, morbidity, developmental outcomes and side effects, however, is still lacking.
Conflict of interest statement
Luz Maria De‐Regil ‐ none known. Maria Elena D Jefferds ‐ none known. Allison C Sylvetsky ‐ none known. Therese Dowswell ‐ none known.
Disclaimer: Luz Maria De‐Regil is a full‐time staff member of the World Health Organization (WHO), Allison C Sylvetsky did a 6‐week internship at WHO (summer 2010), and Therese Dowswell has received financial support from the WHO for her work on this review. Maria Elena Jefferds is a full‐time staff member of the US Centers for Disease Control and Prevention. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the official position, decisions, policy or views of these Organisations.
Figures

















































































































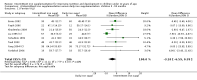

















































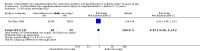









Update of
References
References to studies included in this review
Aguayo 2000 {published data only}
-
- Aguayo VM. School‐administered weekly iron supplementation ‐ effect on the growth and hemoglobin status of non‐anemic Bolivian school‐age children. A randomized placebo‐controlled trial. European Journal of Nutrition 2000;39(6):263‐9. - PubMed
Arcanjo 2011 (C) {published data only}
-
- Arcanjo FPN, Arcanjo CC, Amancio OMS, Braga JAP, Leite AJM. Weekly iron supplementation for the prevention of anemia in pre‐school children: a randomized, double‐blind, placebo‐controlled trial. Journal of Tropical Pediatrics 2011 Feb 1 [Epub ahead of print]. - PubMed
Awasthi 2005 (C) {published data only}
-
- Awasthi S, Verma T, Vir S. Effectiveness of biweekly versus daily iron‐folic acid administration on anaemia status in preschool children. Journal of Tropical Pediatrics 2005;51(2):67‐71. - PubMed
Baqui 2003 {published data only}
-
- Baqui AH, Walker CL, Zaman K, Arifeen S, Chowdhury HR, Wahed MA, et al. Weekly iron supplementation does not block increases in serum zinc due to weekly zinc supplementation in Bangladeshi infants. Journal of Nutrition 2005;135(9):2187‐91. - PubMed
-
- Baqui AH, Zaman K, Persson LA, Arifeen S, Yunus M, Begum N, et al. Simultaneous weekly supplementation of iron and zinc is associated with lower morbidity due to diarrhea and acute lower respiratory infection in Bangladeshi infants. Journal of Nutrition 2003;133(12):4150‐7. - PubMed
-
- Black MM, Baqui AH, Zaman K, Ake Persson L, Arifeen S, Le K, et al. Iron and zinc supplementation promote motor development and exploratory behavior among Bangladeshi infants. American Journal of Clinical Nutrition 2004;80(4):903‐10. - PubMed
-
- Fischer Walker CL, Baqui AH, Ahmed S, Zaman K, Arifeen S, Begum N, et al. Low‐dose weekly supplementation of iron and/or zinc does not affect growth among Bangladeshi infants. European Journal of Clinical Nutrition 2009;63(1):87‐92. - PubMed
Berger 1997 {published data only}
-
- Berger J, Aguayo VM, Téllez W, Luján C, Traissac P, San Miguel JL. Weekly iron supplementation is as effective as 5 day per week iron supplementation in Bolivian school children living at high altitude. European Journal of Clinical Nutrition 1997;51(6):381‐6. - PubMed
Da Silva 2008 {published and unpublished data}
-
- Silva DG, Franceschini Sdo C, Sigulem DM. Growth in non‐anemic infants supplemented with different prophylactic iron doses. Jornal de Pediatria 2008;84(4):365‐72. - PubMed
Desai 2004 (C) {published data only}
-
- Desai MR, Dhar R, Rosen DH, Kariuki SK, Shi YP, Kager PA, et al. Daily iron supplementation is more efficacious than twice weekly iron supplementation for the treatment of childhood anemia in western Kenya. Journal of Nutrition 2004;134(5):1167‐74. - PubMed
Ekvall 2000 {published data only}
-
- Ekvall H, Premji Z, Bjorkman A. Micronutrient and iron supplementation and effective anti‐malarial treatment synergistically improve childhood anaemia. Tropical Medicine and International Health 2000;5(10):696‐705. - PubMed
Engstrom 2008 (C) {published data only}
-
- Engstrom EM, Castro IR, Portela M, Cardoso LO, Monteiro CA. Effectiveness of daily and weekly iron supplementation in the prevention of anemia in infants. Revista de Saúde Pública 2008;42(5):786‐95. - PubMed
Ermis 2002 {published data only}
-
- Ermis B, Demirel F, Demircan N, Gurel A. Effects of three different iron supplementations in term healthy infants after 5 months of life. Journal of Tropical Pediatrics 2002;48(5):280‐4. - PubMed
Evangelista‐Salazar 2004 {published data only}
-
- Evangelista‐Salazar E. Evaluation of the preventive effect of the intermittent provision of iron and vitamin C on the reduction of the iron and neurodevelopment in infants [Evaluacion del efecto preventivo de la administracion intermitente de hierro y vitamina C sobre la disminucion de la reserva de hierro y el neurodesarrollo en lactantes]. Doctoral Thesis. Universidad de Colima, Mexico 2004.
-
- Martínez H, Evangelista Salazar JJ, Avila Jiménez L. Iron supplementation for preventing anaemia in infants [Suplementación con hierro para prevenir anemia en la primera infancia]. In: Luis Durán Arenas, Onofre Muñoz Hernández editor(s). La traducción del conocimiento. del resultado de la investigación a la aplicación de los servicios de salud. México, D.F: CAMS, 2006:71‐86.
Faqih 2006 {published data only}
-
- Faqih AM, Kakish SB, Izzat M. Effectiveness of intermittent iron treatment of two‐ to six‐year‐old Jordanian children with iron‐deficiency anemia. Food and Nutrition Bulletin 2006;27(3):220‐7. - PubMed
Hall 2002 (C) {published and unpublished data}
-
- Hall A, Roschnik N, Ouattara F, Toure I, Maiga F, Sacko M, et al. A randomised trial in Mali of the effectiveness of weekly iron supplements given by teachers on the haemoglobin concentrations of schoolchildren. Public Health Nutrition 2002;5(3):413‐8. - PubMed
Khademloo 2009 {published data only}
-
- Khademloo M, Karami H, Ajami A, Yasari M. Comparison of the effectiveness of weekly and daily iron supplementation in 6‐ to 24‐months‐old babies in urban health centers of Sari, Iran. Pakistan Journal of Biological Sciences 2009;12(2):195‐7. - PubMed
Liu 1995 (C) {published data only}
-
- Liu X‐N, Kang J, Zhao L, Viteri FE. Intermittent iron supplementation in Chinese pre‐schoolchildren is efficient and safe. Food and Nutrition Bulletin 1995;16(2):139‐46.
-
- Liu XNA, Liu PY. The effectiveness of weekly iron supplementation regimen in improving the iron status of Chinese children and pregnant women. Biomedical and Environmental Sciences 1996;9(2‐3):341‐7. - PubMed
Nguyen 2002 {published data only}
-
- Nguyen XN, Berger J, Dao TQ, Nguyen CK, Traissac P, Ha HK. Efficacy of daily and weekly iron supplementation for the control of iron deficiency anaemia in infants in rural Vietnam [Efficacite de la supplementation en fer quotidienne et hebdomadaire pour le controle de l'anemie chez le nourrisson en milieu rural au Vietnam]. Santé (Montrouge, France) 2002;12(1):31‐7. - PubMed
Olsen 2000 {published data only}
-
- Olsen A, Nawiri J, Friis H. The impact of iron supplementation on reinfection with intestinal helminths and Schistosoma mansoni in western Kenya. Transactions of the Royal Society of Tropical Medicine Hygene 2000;94(5):493‐9. - PubMed
-
- Olsen A, Nawiri J, Magnussen P, Krarup H, Friis H. Failure of twice‐weekly iron supplementation to increase blood haemoglobin and serum ferritin concentrations: results of a randomized controlled trial. Annals of Tropical Medicine and Parasitology 2006;100(3):251‐63. - PubMed
Palupi 1997 {published data only}
-
- Palupi L, Schultink W, Achadi E, Gross R. Effective community intervention to improve hemoglobin status in preschoolers receiving once‐weekly iron supplementation. American Journal of Clinical Nutrition 1997;65(4):1057‐61. - PubMed
Roschnik 2003 (C) {unpublished data only}
-
- Roschnik N, Phiri V, Mukaka M. The impact of weekly school‐based iron supplementation. Mangochi District, Malawi (Report). Save the Children, USA February 2003.
Roschnik 2004 (C) {published and unpublished data}
-
- Roschnik N, Parawan A, Baylon MA, Chua T, Hall A. Weekly iron supplements given by teachers sustain the haemoglobin concentration of schoolchildren in the Philippines. Tropical Medicine and International Health 2004;9(8):904‐9. - PubMed
Schultink 1995 {published data only}
-
- Schultink W, Gross R, Gliwitzki M, Karyadi D, Matulessi P. Effect of daily vs twice weekly iron supplementation in Indonesian preschool children with low iron status. American Journal of Clinical Nutrition 1995;61(1):111‐5. - PubMed
Sen 2009 (C) {published and unpublished data}
-
- Sen A, Kanani SJ. Impact of iron‐folic acid supplementation on cognitive abilities of school girls in Vadodara. Indian Pediatrics 2009;46(2):137‐43. - PubMed
-
- Sen A, Kanani SJ. Physical work capacity of young underprivileged school girls impact of daily vs intermittent iron folic acid supplementation: a randomized controlled trial. Indian Pediatrics 2009;46(10):849‐54. - PubMed
Siddiqui 2004 {published data only}
-
- Siddiqui IA, Jaleel A, Rahman MA. Preventive strategy to control iron deficiency anemia in children and adults. Journal of the Pakistan Medical Association 2003;53(4):131‐3. - PubMed
-
- Siddiqui IA, Rahman MA, Jaleel A. Efficacy of daily vs. weekly supplementation of iron in schoolchildren with low iron status. Journal of Tropical Pediatrics 2004;5:276‐8. - PubMed
Sinisterra 1997 (C) {published data only}
-
- Sinisterra OT, Valdes VE, Valverde C. Effect of supplementation with iron salts and knowledge, attitudes and practices in relation to anaemia among schoolchildren in the province of Cocle, Ministry of Health, Republic of Panama (report). [Efecto de la suplementacion con sales de hierro y de conocimientos, actitudes y practicas en relacion a la anemia en escolares de la provincia de Cocle, Republica de Panama: reporte]. Vol. DCE/18, Panama: INCAP, Ministry of Health, Republic of Panama, 1997.
Soemantri 1997 {published data only}
-
- Soemantri AG, Hapsari DE, Susanto JC, Rohadi W, Tamam M, Irawan PW, et al. Daily and weekly iron supplementation and physical growth of school age Indonesian children. Southeast Asian Journal of Tropical Medicine and Public Health 1997;28 Suppl 2:69‐74. - PubMed
Sungthong 2002 {published data only}
-
- Sungthong R, Mo‐Suwan L, Chongsuvivatwong V, Geater AF. Once weekly is superior to daily iron supplementation on height gain but not on hematological improvement among schoolchildren in Thailand. Journal of Nutrition 2002;132(3):418‐22. - PubMed
-
- Sungthong R, Mo‐suwan L, Chongsuvivatwong V, Geater AF. Once‐weekly and 5‐days a week iron supplementation differentially affect cognitive function but not school performance in Thai children. Journal of Nutrition 2004;134(9):2349‐54. - PubMed
Tavil 2003 {published data only}
-
- Tavil B, Sipahi T, Gökçe H, Akar N. Effect of twice weekly versus daily iron treatment in Turkish children with iron deficiency anemia. Pediatric Hematology and Oncology 2003;20(4):319‐26. - PubMed
Taylor 2001 {published data only}
-
- Taylor M, Jinabhai CC, Couper I, Kleinschmidt I, Jogessar VB. The effect of different anthelmintic treatment regimens combined with iron supplementation on the nutritional status of schoolchildren in KwaZulu‐Natal, South Africa: a randomized controlled trial. Transactions of the Royal Society of Tropical Medicine and Hygiene 2001;95(2):211‐6. - PubMed
Thu 1999 {published data only}
-
- Thu BD, Schultink W, Dillon D, Gross R, Leswara ND, Khoi HH. Effect of daily and weekly micronutrient supplementation on micronutrient deficiencies and growth in young Vietnamese children. American Journal of Clinical Nutrition 1999;69(1):80‐6. - PubMed
Verhoef 2002 {published data only}
-
- Verhoef H, West CE, Kraaijenhagen R, Nzyuko SM, King R, Mbandi MM. Malarial anemia leads to adequately increased erythropoiesis in asymptomatic Kenyan children. Blood 2002;15(00(10)):489‐94. - PubMed
-
- Verhoef H, West CE, Nzyuko SM, Vogel S, Valk R, Wanga MA, et al. Intermittent administration of iron and sulfadoxine‐pyrimethamine to control anaemia in Kenyan children: a randomised controlled trial. Lancet 2002;21(360(9337)):908‐14. - PubMed
Yang 2004 (C) {published data only}
-
- Yang Q, Yin S, Zhao X, An J. Effect of daily or once weekly iron supplementation on growth and iron status of preschool children. Wei sheng yan jiu = Journal of Hygiene Research 2004;33(2):205‐7. - PubMed
Young 2001 {published data only}
-
- Young MW. The effectiveness of weekly iron and vitamin supplementation of Malawian preschool children. South African Medical Journal = Suid‐Afrikaanse tydskrif vir geneeskunde 2001;91(1):49‐50. - PubMed
Yurdakok 2004 {published data only}
-
- Yurdakök K, Temiz F, Yalçin SS, Gümrük F. Efficacy of daily and weekly iron supplementation on iron status in exclusively breast‐fed infants. Journal of Pediatric Hematology/Oncology 2004;26(5):284‐8. - PubMed
References to studies excluded from this review
Agarwal 2003 {published data only}
-
- Agarwal KN, Gomber S, Bisht H, Som M. Anemia prophylaxis in adolescent school girls by weekly or daily iron‐folate supplementation. Indian Pediatrics 2003;40(4):296‐301. - PubMed
Ahmed 2001 {published data only}
-
- Ahmed F, Khan MR, Jackson AA. Concomitant supplemental vitamin A enhances the response to weekly supplemental iron and folic acid in anemic teenagers in urban Bangladesh. American Journal of Clinical Nutrition 2001;74(1):108‐15. - PubMed
Ahmed 2005 {published data only}
-
- Ahmed F, Khan MR, Akhtaruzzaman M, Karim R, Marks GC, Banu CP, et al. Efficacy of twice‐weekly multiple micronutrient supplementation for improving the hemoglobin and micronutrient status of anemic adolescent schoolgirls in Bangladesh. American Journal of Clinical Nutrition 2005;82(4):829‐35. - PubMed
Avila‐Jimenez 2011 {unpublished data only}
-
- Ávila Jiménez L, Méndez, Villalpando‐Hernández S, Martínez‐Salgado H. Preventive iron supplementation and nutritional status of infants measured by anthropometry assigned to IMSS obligatory regimen [Suplementación preventiva con hierro y estado nutricio medido por antropometría de lactantes del régimen obligatorio del IMSS]. Unpublished article sent by Dr Homero Martinez.
-
- Ávila‐Jiménez L, Méndez I, Villalpando‐Hernández S, Martínez‐Salgado H. Effect of iron supplementation on growth velocity in infants [Efecto de una suplementación con hierro sobre la velocidad de crecimiento en lactantes]. Unpublished article sent by Dr Homero Martinez.
Azeredo 2010 {published data only}
-
- Azeredo CM, Cotta RM, Sant'Ana LF, Franceschini Sdo C, Ribeiro Rde C, Lamounier JA, et al. Greater effectiveness of daily iron supplementation scheme in infants [Efectividad superior del esquema diario de suplementación de hierro en lactantes]. Revista de Saúde Pública 2010;44(2):230‐9. - PubMed
Beasley 2000 {published data only}
-
- Beasley NM, Tomkins AM, Hall A, Lorri W, Kihamia CM, Bundy DA. The impact of weekly iron supplementation on the iron status and growth of adolescent girls in Tanzania. Tropical Medicine and International Health 2000;5(11):794‐9. - PubMed
Briars 2003 {published data only}
-
- Adank C, Green TJ, Skeaff CM, Briars B. Weekly high‐dose folic acid supplementation is effective in lowering serum homocysteine concentrations in women. Annals of Nutrition and Metabolism 2003;47(2):55‐9. - PubMed
Februhartanty 2002 {published data only}
-
- Februhartanty J, Dillon D, Khusun H. Will iron supplementation given during menstruation improve iron status better than weekly supplementation?. Asia Pacific Journal of Clinical Nutrition 2002;11(1):36‐41. - PubMed
Hafeez 1998 {published data only}
-
- Hafeez A, Ahmad P. Iron deficiency anaemia: continuous versus intermittent treatment in anaemic children. Journal of the Pakistan Medical Association 1998;48(9):269‐72. - PubMed
Hop 2005 {published data only}
-
- Hop LT, Berger J. Multiple micronutrient supplementation improves anemia, micronutrient nutrient status, and growth of Vietnamese infants: double‐blind, randomized, placebo‐controlled trial. Journal of Nutrition 2005;135(3):660s‐5s. - PubMed
Jackson 2003 {published data only}
-
- Jackson RT, Al‐Mousa Z, Al‐Raqua M, Prakash P. Effect of short‐term weekly supplementation on anemia and symptoms in adolescent Kuwaiti girls. Kuwait Medical Journal 2003;35(4):275‐80.
Jaleel 2004 {published data only}
-
- Jaleel A, UrRahman MA. Daily iron supplementation versus intermittent dose. Saudi Medical Journal 2004;25(8):1124‐5. - PubMed
Jayatissa 1999 {published data only}
-
- Jayatissa R, Piyasena P. Adolescent schoolgirls: daily or weekly iron supplementation. Food and Nutrition Bulletin 1999;20(4):429‐34.
Kanal 2005 {published data only}
-
- Kanal K, Busch‐Hallen J, Cavalli‐Sforza T, Crape B, Smitasiri S, Cambodian Weekly Iron‐Folic Acid Program Team. Weekly iron‐folic acid supplements to prevent anemia among Cambodian women in three settings: process and outcomes of social marketing and community mobilization. Nutrition Reviews 2005;63(12 Pt 2):S126‐33. - PubMed
Kapur 2003 {published data only}
-
- Kapur D, Sharma S, Agarwal KN. Effectiveness of nutrition education, iron supplementation or both on iron status in children. Indian Pediatrics 2003;40(12):1131‐44. - PubMed
Kianfar 2000 {published data only}
-
- Kianfar H, Kimiagar M, Ghaffarpour M. Effect of daily and intermittent iron supplementation on iron status of high school girls. International Journal for Vitamin and Nutrition Research 2000;70(4):172‐7. - PubMed
Lechtig 2006 {published data only}
-
- Lechtig A, Gross R, Vivanco OA, Gross U, López de Romaña D. Lessons learned from the scaling‐up of a weekly multimicronutrient supplementation program in the integrated food security program (PISA). Food and Nutrition Bulletin 2006;27 Suppl Peru(4):160‐5. - PubMed
Leenstra 2009 {published data only}
-
- Leenstra T, Kariuki SK, Kurtis JD, Oloo AJ, Kager PA, Ter Kuile FO. The effect of weekly iron and vitamin A supplementation on hemoglobin levels and iron status in adolescent schoolgirls in western Kenya. European Journal of Clinical Nutrition 2009;63(2):173‐82. - PubMed
Lima 2006 {published data only}
-
- Lima AC, Lima MC, Guerra MQF, Romani SAM, Eickmann SH, Lira PIC. Impact of weekly treatment with ferrous sulfate on hemoglobin level, morbidity and nutritional status of anemic infants. Jornal de Pediatria 2006;82(6):452‐7. - PubMed
Lin 2001 {published data only}
-
- Lin X, Tang Y, Long Z. Effects of vitamin A and iron supplementation on the improvement of iron status and immunological function in preschool children. Chinese Journal of Preventive Medicine ‐ Zhonghua Yu Fang Yi Xue Za Zhi 2001;35(6):374‐7. - PubMed
López de Romaña 2005 {published data only}
-
- López de Romaña G, Cusirramos S, López de Romaña D, Gross R. Efficacy of multiple micronutrient supplementation for improving anemia, micronutrient status, growth, and morbidity of Peruvian infants. Journal of Nutrition 2005;135(3):646s‐52s. - PubMed
López de Romaña 2006 {published data only}
-
- López de Romaña D, Verona S, Vivanco OA, Gross R. Protective effect of multimicronutrient supplementation against anemia among children, women, and adolescent girls in lower‐income areas of Chiclayo, Peru. Food and Nutrition Bulletin 2006;27 Suppl Peru(4):S143‐50. - PubMed
Menendez 1997 {published data only}
-
- Menendez C, Kahigwa E, Hirt R, Vounatsou P, Aponte JJ, Font F, et al. Randomised placebo‐controlled trial of iron supplementation and malaria chemoprophylaxis for prevention of severe anaemia and malaria in Tanzanian infants. Lancet 1997;350(9081):844‐50. - PubMed
Mwanakasale 2009 {published data only}
Perrin 2002 {published data only}
-
- Perrin E, Rothman R, Coyne‐Beasley T, Ford C, Bordley WC. Is weekly iron and folic acid supplementation as effective as daily supplementation for decreasing incidence of anemia in adolescent girls?. Archives of Pediatrics and Adolescent Medicine 2002;156(2):128‐30. - PubMed
Risonar 2008 {published data only}
-
- Risonar MG, Tengco LW, Rayco‐Solon P, Solon FS. The effect of a school‐based weekly iron supplementation delivery system among anemic schoolchildren in the Philippines. European Journal of Clinical Nutrition 2008;62(8):991‐6. - PubMed
Rivera 1998 {published data only}
-
- Rivera G, Thomson M. Effect of ferrous salts supplementation on iron status of schoolchildren, Chiriqui, Panama, 1995‐1997 [Efecto de la suplementacion con sales ferrosas en el estado nutricional de hierro de escolares, Chiriqui, Panama, 1995‐1997]. Panama: Ministry of Health, Republic of Panama, 1998.
Schümann 2009 {published data only}
-
- Schümann K, Longfils, P, Monchy D, Xylander S, Weinheimer H, Solomons NW. Efficacy and safety of twice‐weekly administration of three RDAs of iron and folic acid with and without complement of 14 essential micronutrients at one or two RDAs: a placebo‐controlled intervention trial in anemic Cambodian infants 6 to 24 months of age. European Journal of Clinical Nutrition 2009;63(3):355‐68. - PubMed
Shah 2002 {published data only}
-
- Shah BK, Gupta P. Weekly vs daily iron and folic acid supplementation in adolescent Nepalese girls. Archives of Pediatrics and Adolescent Medicine 2002;156(2):131‐5. - PubMed
Sharma 2000 {published data only}
-
- Sharma A, Prasad K, Rao KV. Identification of an appropriate strategy to control anemia in adolescent girls of poor communities. Indian Pediatrics 2000;37(3):261‐7. - PubMed
Shobha 2003 {published data only}
-
- Shobha S, Sharada D. Efficacy of twice weekly iron supplementation in anemic adolescent girls. Indian Pediatrics 2003;40(12):1186‐90. - PubMed
Smuts 2005 {published data only}
-
- Smuts CM, Dhansay MA, Faber M, Stuijvenberg ME, Swanevelder S, Gross R, et al. Efficacy of multiple micronutrient supplementation for improving anemia, micronutrient status, and growth in South African infants. Journal of Nutrition 2005;135(3):653s‐9s. - PubMed
Soekarjo 2004 {published data only}
-
- Soekarjo DD, Pee S de, Kusin JA, Schreurs WH. Schultink W, Muhilal, et al. Effectiveness of weekly vitamin A (10,000 IU) and iron (60 mg) supplementation for adolescent boys and girls through schools in rural and urban East Java, Indonesia. European Journal of Clinical Nutrition 2004;58(6):927‐37. - PubMed
Sotelo‐Cruz 2002 {published data only}
-
- Sotelo‐Cruz N, Gómez‐Rivera N, Ferrá‐Fragoso S, Pereyda‐Galaz DE. Ferrous sulfate weekly dose for iron deficiency in preschool children. Gaceta Médica de México 2002;138(3):225‐30. - PubMed
Tee 1999 {published data only}
-
- Tee ES, Kandiah M, Awin N, Chong SM, Satgunasingam N, Kamarudin L, et al. School‐administered weekly iron‐folate supplements improve hemoglobin and ferritin concentrations in Malaysian adolescent girls. American Journal of Clinical Nutrition 1999;69(6):1249‐56. - PubMed
Tomashek 2001 {published data only}
-
- Tomashek KM, Woodruff BA, Gotway CA, Bloland P, Mbaruku G. Randomized intervention study comparing several regimens for the treatment of moderate anemia among refugee children in Kigoma Region,Tanzania. American Journal Tropical Medicine and Hygiene 2001;64(3‐4):164‐71. - PubMed
UNICEF 2006 {published data only}
-
- Ministry of Health Panama, UNICEF, PAHO. [Situacion de deficiencia de hierro y anemia]. Status of iron deficiency and anaemia. Panama: UNICEF, 2006.
Vir 2008 {published data only}
-
- Vir SC, Singh N, Nigam AK, Jain R. Weekly iron and folic acid supplementation with counselling reduces anemia in adolescent girls: a large‐scale effectiveness study in Uttar Pradesh, India. Food and Nutrition Bulletin 2008;29(3):186‐94. - PubMed
Wijaya‐Erhardt 2007 {published data only}
-
- Wijaya‐Erhardt M, Erhardt JG, Untoro J, Karyadi E, Wibowo L, Gross R. Effect of daily or weekly multiple‐micronutrient and iron foodlike tablets on body iron stores of Indonesian infants aged 6‐12 mo: a double‐blind, randomized, placebo‐controlled trial. American Journal of Clinical Nutrition 2007;86(6):1680‐6. - PubMed
Zavaleta 2000 {published data only}
-
- Zavaleta N, Respicio G, Garcia T. Efficacy and acceptability of two iron supplementation schedules in adolescent school girls in Lima, Peru. Journal of Nutrition 2000;130(2):462s‐4s. - PubMed
References to studies awaiting assessment
Husseini 1999 {unpublished data only}
-
- Husseini T. Bagor, Indonesia Trial. Information included in Beaton 1999.
Kargarnovin 2010 {published data only}
-
- Kargarnovin Z, Tamkins A, Jalaly M, Salavan K, Pam L. Effects of daily versus weekly iron therapy in infants between 6‐24 months for iron deficiency anemia. Journal of Nursing and Midwifery 2010;20(69):48.
Reid 2001 {published data only}
-
- Reid ED, Lopez P, Galaviz IA, Isoard F, Rosado JL, Allen LH. Hematological and biochemical responses of rural Mexican preschoolers to iron alone or iron plus micronutrients. FASEB Journal. 2001; Vol. 15:A731.
References to ongoing studies
Zeeba Zaka‐ur‐Rab 2010 {published data only}
-
- Dr. Zeeba Zaka‐ur‐Rab. A clinical trial to compare the effects of daily versus intermittent iron supplementation on markers of oxidative stress and anti‐oxidant status in children with iron deficiency anemia . The Clinical Trials Registry‐ India (CTRI).
Additional references
ACC/SCN 1991
-
- Administrative Committee on Coordination/Subcommittee on Nutrition (United Nations). Controlling iron deficiency. ACC/SCN State of the Art Series. Vol. Nutrition Policy Discussion Paper No 9, Geneva: United Nations Administrative Committee on Coordination: Subcommittee on Nutrition, 1991.
Adetifa 2009
Balshem 2010
-
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. - PubMed
Baqui 2005
-
- Baqui AH, Fischer Walker CL, Zaman K, Arifeen SE, Chowdhury HR, Wahed MA, et al. Weekly iron supplementation does not block increases in serum zinc due to weekly zinc supplementation in Bangladeshi infants. Journal of Nutrition 2005;135(9):2187‐91. - PubMed
Beard 2008
Beaton 1999
-
- Beaton GH, McCabe GP. Efficacy of intermittent iron supplementation in the control of iron deficiency anaemia in developing countries. An analysis of experience (final report). Ottawa: The Micronutrient Initiative, 1999.
Bhutta 2009
-
- Bhutta Z, Klemm R, Shahid F, Rizvi A, Rah JH, Christian P. Treatment response to iron and folic alone is the same as with multivitamins and/or anthelminthics in severely anemic 6 to 24‐month‐old children. Journal of Nutrition 2009;139(8):1568. - PubMed
Borenstein 2008
-
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta‐Analysis (Statistics in Practice). Chichester (UK): John Wiley & Sons, 2008.
Bothwell 2000
-
- Bothwell TH. Iron requirements in pregnancy and strategies to meet them. American Journal of Clinical Nutrition 2000;72(1):257S‐64S. - PubMed
Casanueva 2003
-
- Casanueva E, Viteri FE. Iron and oxidative stress in pregnancy. Journal of Nutrition 2003;133(5):1700S‐8S. - PubMed
Cole 2010
Davidsson 2003
-
- Davidsson L. Approaches to improve iron bioavailability from complementary foods. Journal of Nutrition 2003;133(5):1560S‐2S. - PubMed
De‐Regil 2011
-
- De‐Regil LM, Pena‐Rosas JP, Flores‐Ayala R, Jefferds MED. WHO/CDC logic model for micronutrient interventions in public health. FASEB Journal March 17, 2011;25:108.1.
Fernández‐Gaxiola 2011
Gera 2007
-
- Gera T, Sachdev HP, Nestel P, Sachdev SS. Effect of iron supplementation on haemoglobin response in children: systematic review of randomised controlled trials. Journal of Pediatric Gastroenterology and Nutrition 2007;44(4):468‐86. - PubMed
GRADEpro 2008 [Computer program]
-
- Jan Brozek, Andrew Oxman, Holger Schünemann. GRADEpro Version 3.2 for Windows. Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group, 2008.
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2011.
Iannoti 2006
Lozoff 2000
-
- Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics 2000;105(4):e51. - PubMed
Lozoff 2007
-
- Lozoff B. Iron deficiency and child development. Food and Nutrition Bulletin 2007;28(4):S560‐71. - PubMed
Mills 2009
-
- Mills RJ, Davies MW, McGuire W. Iron supplementation in enterally fed preterm infants. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD005095] - DOI
Moy 2006
-
- Moy RJD. Prevalence, consequences, and prevention of childhood nutritional iron deficiency: a child public health perspective. Clinical and Laboratory Haematology 2006;28(5):291‐8. - PubMed
NIH 2011
-
- NIH Iron and Malaria Technical Working Group. Chapter 2: Mechanisms. In: Raiten D, Namaste S, Brabin B editor(s). Considerations for the Safe and Effective use of Iron Interventions. Bethesda: Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), 2011 (in press):16‐51.
Okebe 2011
Olsen 2006
-
- Olsen A, Nawiri J, Magnussen P, Krarup H, Friis H. Failure of twice weekly iron supplementation to increase blood haemoglobin and serum ferritin concentrations: results of a randomized controlled trial. Annals of Tropical Medicine and Parasitology 2006;100(3):251‐63. - PubMed
Peña‐Rosas 2009
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Simmons 1993
-
- Simmons. Evaluation of a gastric delivery system for iron supplementation in pregnancy. American Journal of Clinical Nutrition 1993;58(5):622‐6. - PubMed
Stoltzfus 2011
-
- Stoltzfus RJ. Iron interventions for women and children in low‐income countries. Journal of Nutrition 2011;141(4):756S–62S. - PubMed
Thu 1999
-
- Thu BD, Schultink W, Dillon D, Gross R, Dhevita Leswara N, Khoi HH. Effect of daily and weekly micronutrient supplementation on micronutrient deficiencies and growth in young Vietnamese children. American Journal of Clinical Nutrition 1999;69(1):80‐6. - PubMed
Viteri 1997
-
- Viteri FE. Iron supplementation for the control of iron deficiency in populations at risk. Nutrition Reviews 1997;55(6):195‐209. - PubMed
Viteri 2005
-
- Viteri FE, Berger J. Importance of pre‐pregnancy and pregnancy iron status: can long‐term weekly preventive iron and folic acid supplementation achieve desirable and safe status?. Nutrition Reviews 2005;63(12 Pt 2):S65‐76. - PubMed
WHO 2001
-
- WHO, UNICEF, UNU. Iron Deficiency Anaemia Assessment, Prevention and Control: A Guide for Programme Managers. 1st Edition. Geneva: World Health Organization, 2001:1‐114.
WHO 2009
-
- World Health Organization. Weekly iron and folic acid supplementation programmes for women of reproductive age: An analysis of best programme practices (WHO Position Statement). Manila: World Health Organization Regional Office for the Western Pacific, 2009.
WHO 2009a
-
- World Health Organization. Infant and young child feeding: model chapter for textbooks for medical students and allied health professionals. Geneva: World Health Organization (http://whqlibdoc.who.int/publications/2009/9789241597494_eng.pdf). Geneva: World Health Organization, 2009. - PubMed
WHO 2009b
-
- World Health Organization. Weekly iron folic acid supplementation (WIFS) in women of reproductive age: its role in promoting optimal maternal and child health. Geneva: World Health Organization, 2009. - PubMed
WHO 2011a
-
- World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization, 2011.
WHO 2011b
-
- WHO. Serum ferritin concentrations for the assessment of iron status and iron deficiency in populations. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization, 2011.
WHO/CDC 2008
-
- World Health Organization/Centers for Disease Control and Prevention (USA). Worldwide prevalence of anaemia 1993‐2005. In: Benoist B, McLean E, Egli I, Cogswell M editor(s). WHO Global Database on Anaemia. Geneva: World Health Organization, 2008.
WHO/CDC 2011
-
- World Health Organization, Centers for Disease Control and Prevention. Logic model for micronutrient interventions in public health. Vitamin and Mineral Nutrition Information System. (WHO/NMH/NHD/MNM/11.5) (http://www.who.int/vmnis/toolkit/WHO‐CDC‐english_colour.pdf, accessed 7 July 2011).. Geneva: World Health Organization, 2011.
Wright 1990
-
- Wright AJ, Southon S. The effectiveness of various iron supplementation regimens in improving the Fe status of anemic rats. British Journal of Nutrition 1990;63(3):579–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

