Consensus paper: roles of the cerebellum in motor control--the diversity of ideas on cerebellar involvement in movement
- PMID: 22161499
- PMCID: PMC4347949
- DOI: 10.1007/s12311-011-0331-9
Consensus paper: roles of the cerebellum in motor control--the diversity of ideas on cerebellar involvement in movement
Abstract
Considerable progress has been made in developing models of cerebellar function in sensorimotor control, as well as in identifying key problems that are the focus of current investigation. In this consensus paper, we discuss the literature on the role of the cerebellar circuitry in motor control, bringing together a range of different viewpoints. The following topics are covered: oculomotor control, classical conditioning (evidence in animals and in humans), cerebellar control of motor speech, control of grip forces, control of voluntary limb movements, timing, sensorimotor synchronization, control of corticomotor excitability, control of movement-related sensory data acquisition, cerebro-cerebellar interaction in visuokinesthetic perception of hand movement, functional neuroimaging studies, and magnetoencephalographic mapping of cortico-cerebellar dynamics. While the field has yet to reach a consensus on the precise role played by the cerebellum in movement control, the literature has witnessed the emergence of broad proposals that address cerebellar function at multiple levels of analysis. This paper highlights the diversity of current opinion, providing a framework for debate and discussion on the role of this quintessential vertebrate structure.
Conflict of interest statement
Figures
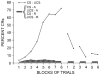








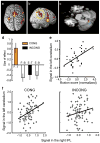

References
-
- Baier B, Stoeter P, Dieterich M. Anatomical correlates of ocular motor deficits in cerebellar lesions. Brain. 2009;132:2114–24. - PubMed
-
- Ohki M, Kitazawa H, Hiramatsu T, Kaga K, Kitamura T, Yamada J, Nagao S. Role of primate cerebellar hemisphere in voluntary eye movement control revealed by lesion effects. J Neurophysiol. 2009;101(2):934–47. - PubMed
-
- Hiramatsu T, Ohki M, Kitazawa H, Xiong G, Kitamura T, Yamada J, Nagao S. Role of primate cerebellar lobulus petrosus of paraflocculus in smooth pursuit eye movement control revealed by chemical lesion. Neurosci Res. 2008;60(3):250–8. - PubMed
-
- Zee DS, Leigh RJ, Mathieu-Millaire F. Cerebellar control of ocular gaze stability. Ann Neurol. 1980;7:37–40. - PubMed
-
- Zee DS, Yamazaki A, Butler PH, Gücer G. Effects of ablation of flocculus and paraflocculus of eye movements in primate. J Neurophysiol. 1981;46:878–99. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

